Menu
A laser is an intense light source that is coherent, directional, and monochromatic. This means that the phases of the light waves are aligned, the waves travel in a single direction, and they emit a narrow range of wavelengths1 .
This is different to other light sources, such as lamps or light-emitting diodes (LEDs), which are typically less intense, non-coherent (phases of light waves are out of sync with each other), and emit a wider range of wavelengths in many directions (Figure 1). 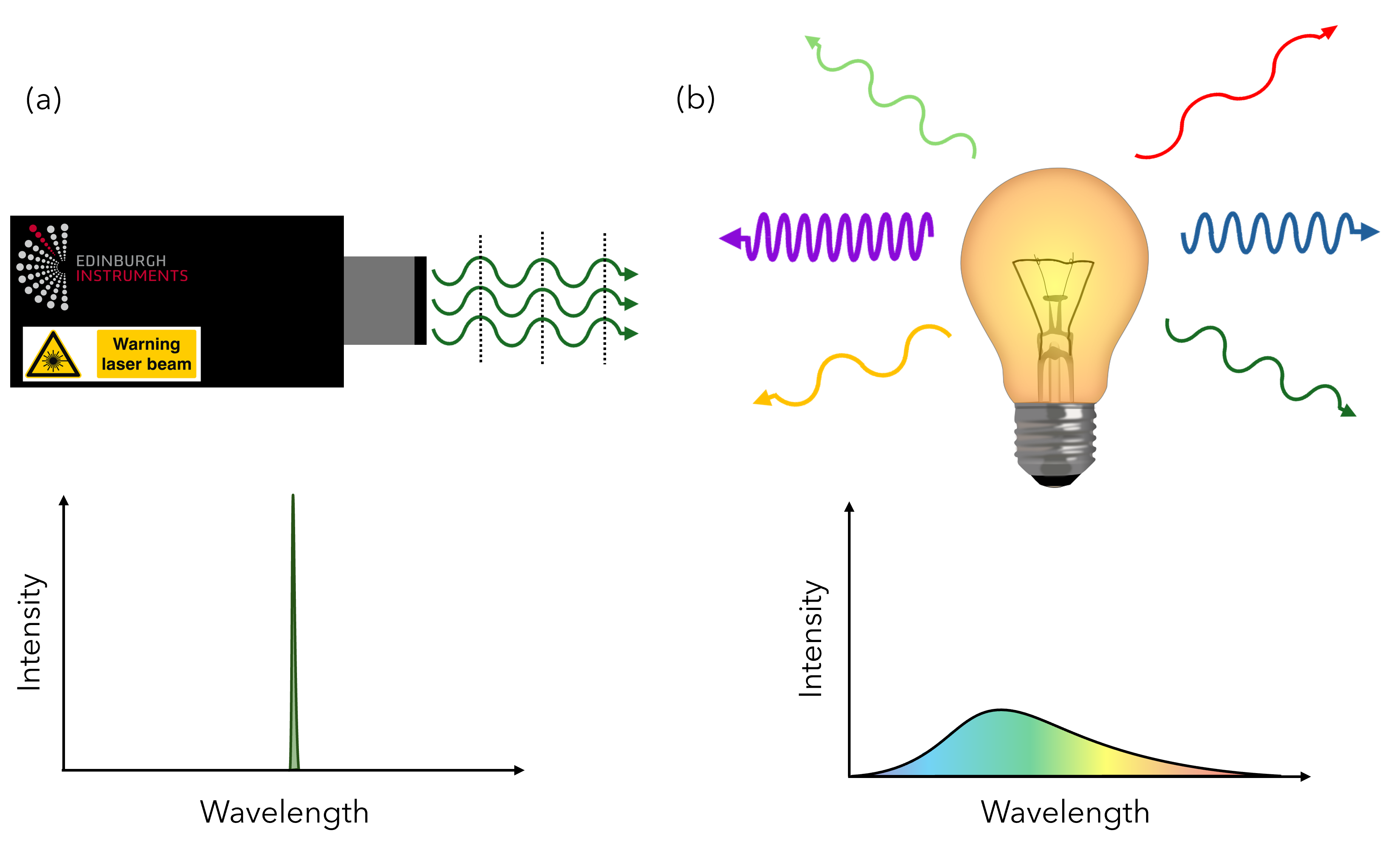
Figure 1: Differences between lasers and standard light sources (a) Coherent and directional emission from a laser source. The dashed lines indicate the alignment of phases of the light waves. Lasers typically emit more intense light with a narrower range of wavelengths. (b) Non-coherent and non-directional emission of light from a lamp. These types of light sources can emit a wide range of wavelengths.
Lasers can be categorised as continuous wave (CW) or pulsed based on the duration of light emission. CW lasers output a constant beam, whereas pulsed lasers output a periodic pulse, and can achieve higher intensities. The width of the pulse may range from seconds to femtoseconds (10-15 s). The repetition rate describes how many pulses fire per second, in Hz.
Lasers work by stimulated emission, one of three main processes that Einstein used to describe how matter exchanges energy with electromagnetic radiation, or light. This process is what gave rise to the word “laser”, which is an acronym meaning Light Amplification by Stimulated Emission of Radiation2 .
To explain this concept, we can consider an atom that has a ground energy level, E1, and a higher energy level, E2. We can call the difference in the energy between these levels ΔE (Figure 2).
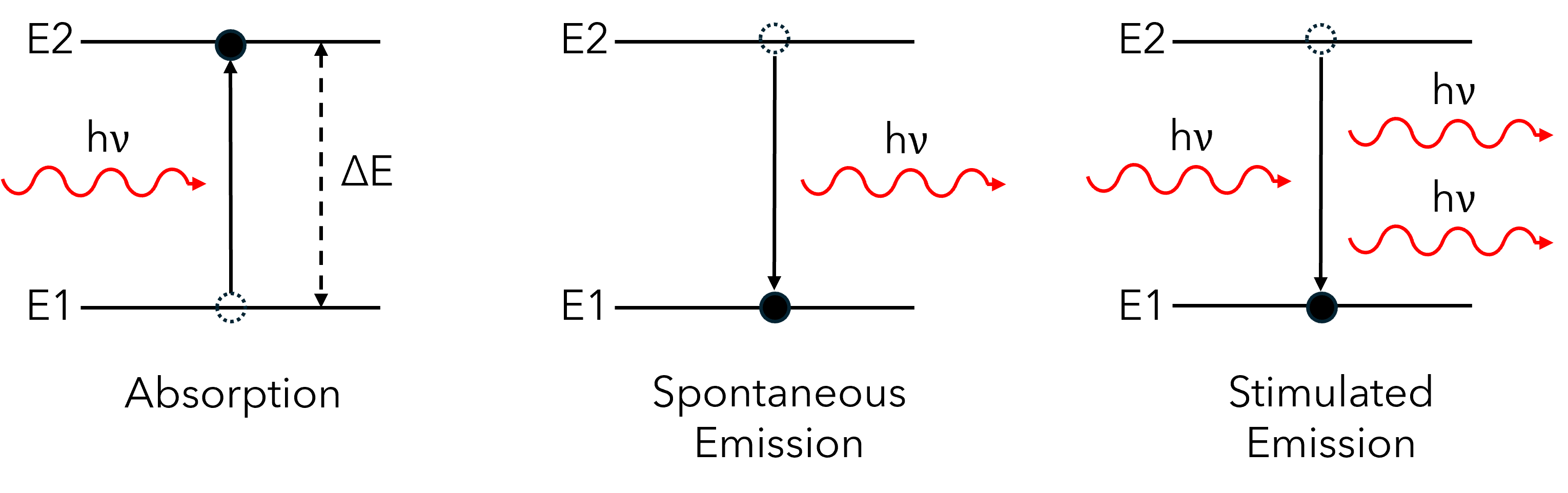
Figure 2: The three main processes that may occur when atoms exchange energy with light. Spontaneous and stimulated emission are classed as radiative processes (as they involve the emission of radiation, or light).
To achieve lasing, the rate of stimulated emission needs to be higher than the rate of absorption. For this to happen, the number of atoms (N), or population, in a higher energy state needs to be greater than the population in the ground state. This is known as population inversion. At thermal equilibrium, inversion is disfavoured, so we need to provide the material with energy to populate the higher energy states until N2 > N1. This is known as “pumping” and may be achieved by irradiation with a lamp, another laser, or by electrical current.
In a two-energy level system it is not possible to achieve population inversion. This is because the pumping energy is equally likely to stimulate emission as it is to be absorbed, which at best will result in N1 = N2. As such, at least three levels are required.
In a three-energy level system, the following stages occur (Figure 3):
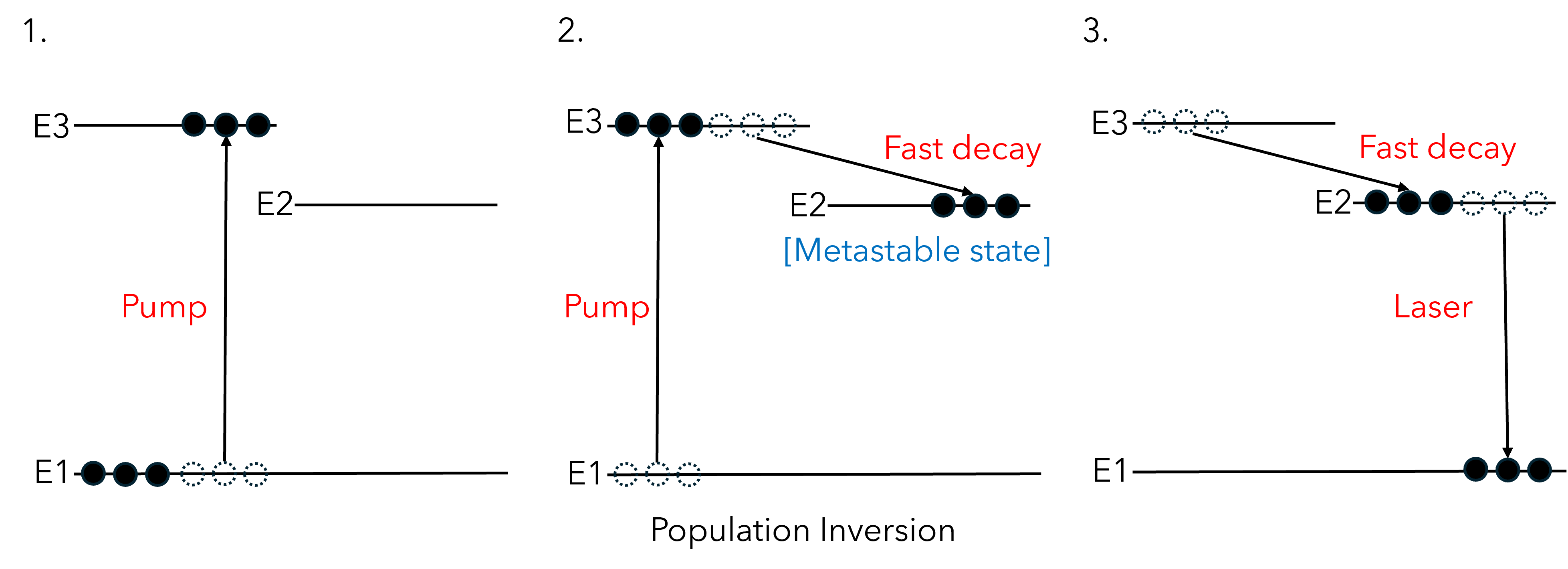
Figure 3: The stages of generating laser emission with a three-energy level laser system. Population of higher energy states with respect to the ground state (N2,3 > N1) results in population inversion.
Because the pumping and stimulated emission photons are of different energies, the pump does not induce stimulated emission. Also, the metastable state ensures that population of E2 and E3 will be greater than E1 (N2,3> N1), resulting in population inversion! Four-level systems also exist and work under a similar principle.
All lasers comprise three essential components: a gain medium, a pump, and a cavity3 (Figure 4):
Table 1: Essential components of a laser and their functions
| Component | Function |
|---|---|
| Gain Medium | A material that is capable of stimulated emission to produce laser light. This can be a solid, liquid, or gas. |
| Pump | A source of energy to induce a population inversion. This can be a light source, such as another laser or a lamp, or an electrical source. |
| Cavity | Chamber with reflectors (mirrors) that recirculate laser photons generated by stimulated emission to induce further stimulated emission. One mirror is a total reflector, and the other a partial reflector which releases a portion of the light as the output laser beam. |
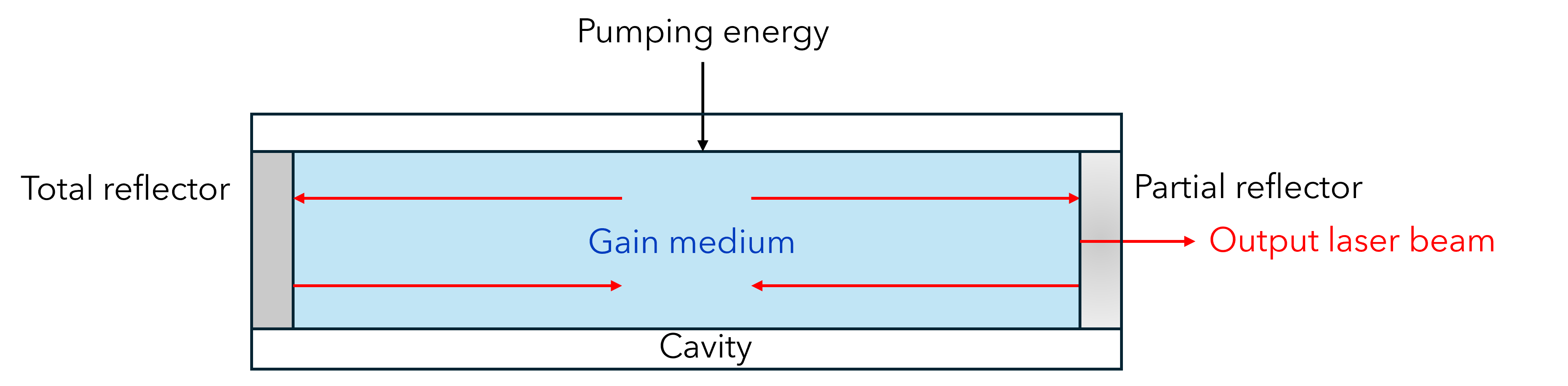
Figure 4: Schematic of a typical laser. Pump energy raises the gain medium to higher energy state, where a population inversion is reached. Light generated by stimulated emission bounces back and forth in the laser cavity, reflected by mirrors at either end, self-stimulating more emission. The laser beam is released via partially reflective mirror.
To start the laser, the following steps occur:
There are several types of different lasers that can be used for a wide range of applications. A short list of some well-known lasers and some of their uses is given below (Figure. 5)4 :
Solid-state lasers:
Gas-state lasers:
Dye lasers:
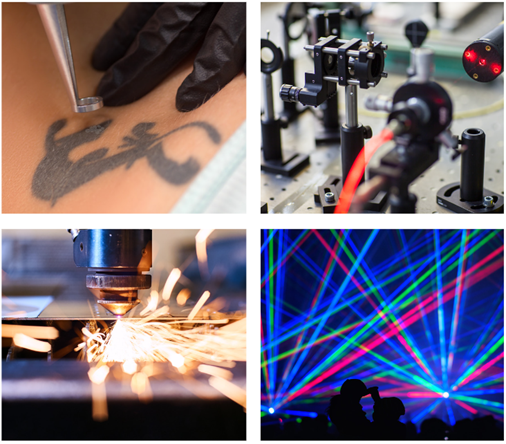 Figure 5: Applications of lasers: Tattoo removal (top left), spectroscopy and research (top right), laser cutting (bottom left), laser light shows (bottom right).
Figure 5: Applications of lasers: Tattoo removal (top left), spectroscopy and research (top right), laser cutting (bottom left), laser light shows (bottom right).
At Edinburgh Instruments, we produce lasers for spectroscopy. This includes CO2 lasers, our range of pulsed solid state lasers, and our picosecond wavelength tunable AGILE laser (Figure 6).
We also integrate pulsed Nd:YAG lasers into our LP980 spectrometers for transient absorption and phosphorescence spectroscopy, as well as Ti:Sa lasers into our FLS1000 spectrometers for fluorescence spectroscopy. CW laser diodes are used in our RMS1000 and RM5 Raman microscopes.

Figure 6: Edinburgh Instruments’ pulsed diode EPL lasers (top), the PL5 CO2 laser (bottom left), and picosecond tunable wavelength white light AGILE laser (bottom right).
If you are interested in find out more about our lasers, get in touch with our friendly team by completing this form.