Zero-dimensional (0D) metal halide hybrids are an emerging class of semiconducting materials for optoelectronic applications including light-emitting diodes, lasers and photovoltaics. Heterometallic (comprising of more than one type of metal centre) metal halide hybrids, in particular, hold great promise due to the potential for synergistic photo-physical properties depending on the choice of the constituent metal centres.1 In this application note time-resolved emission spectroscopy (TRES) with the FLS1000 Photoluminescence Spectrometer is used to identify the emission pathways in the 0D metal halide hybrid, Tris SbMnCl.
Tris SbMnCl was prepared by researchers at the Indian Institute of Science Education and Research (IISER) Tirupati and full synthesis details can be found in the accompanying publication.1 The photoluminescence properties were characterised using an Edinburgh Instruments FLS1000 Photoluminescence spectrometer equipped with a VPLED310 Variable Pulse Width Light Emitting Diode and Multichannel Scaling (MCS) Lifetime Electronics.

Figure 1: FLS1000 Photoluminescence Spectrometer.
The Tris SbMnCl was excited with the VPLED310 source in CW mode. The VPL/VPLED series are pulsed diode lasers and LEDs with a user-settable pulse width (50 ns to 1 ms) in addition to a CW mode that provides continuous excitation for spectral measurements. The emission spectrum of Tris SbMnCl, when excited at 310 nm, is shown in Figure 2, revealing a bright emission centred at 635 nm. To identify which metal centres are contributing to this emission, time-resolved emission spectroscopy (TRES) can be utilised

Figure 2: Emission spectrum of Tris SbMnCl.
In TRES, a pulsed excitation source excites the sample, and the resulting photoluminescence decay is recorded sequentially as a function of emission wavelength to create a 3D dataset that contains the spectral and time evolution of the photoluminescence. The Fluoracle® operating software of the FLS1000 enables automated TRES spectra to be acquired using either TCSPC or MCS mode for measuring short and long lifetimes respectively.
To acquire the TRES of Tris SbMnCl the VPLED310 was set to a pulse width of 100 ns and a repetition rate of 10 Hz. The photoluminescence decays were acquired using MCS mode over a wavelength range of 400 to 900 nm in 4 nm steps. Two TRES profiles of Tris SbMnCl were measured, the first focusing on the 0-20 µs time range with a time-resolution of 50 ns (Figure 3a & 3b); and a second over a time range of 50 ms with a lower time-resolution of 10 µs (Figure 3c & d) to capture the long timescale behaviour.

Figure 3: TRES of Tris SbMnCl acquired over (a) a 20 µs time range and (b) a 50 ms time range. Extracted decays at λem = 650 nm are shown in panels (b) & (d).
Looking first at the short timescale behaviour in Figures 3a & 3b, upon excitation there is a prompt emission channel that decays within the first 20 ns followed by an intermediate channel that decays over the next ~7 µs (τ= 904 ns). Both processes emit broadly over the measured wavelength range. This is followed by a slower emission channel which appears static in the 20 µs time range of Figures 3a & 3b but can be clearly seen within the 50 ms time range of Figures 3c & 3d as a decay lasting ~40 ms (τ= 6.01 ms). The ms channel is notably narrower than the ns & µs channels, emitting over a 550 nm to 800 nm wavelength range.
The spectra associated with each emission channel can be obtained by integrating the corresponding time range of the TRES profile in Fluoracle, and the normalised spectra of the three channels are shown in Figure 4a. The ns and µs channel spectra are broad spanning 400 nm to 900 nm with the ns channel blue-shifted with respect to the µs. This broad emission on a ns and µs timescale is characteristic of Sb emission in 0D chloride hybrids.2 When excited with high energy photons (~ 300 nm) both the singlet and triplet self-trapped exciton (STE) manifolds in 0D Sb chlorides are populated. The radiative transition of the singlet and triplet STEs leads to a higher energy fluorescence emission on a ns and a lower energy phosphorescence emission on a µs timescale respectively which agrees with Figure 4a, and the ns & µs emission channels can therefore be assigned to emissive Sb centres in Tris SbMnCl. The ms timescale emission centred at 635 nm is also observed in the parent compound Tris MnCl (no Sb doping) and is distinctive of Mn2+centre emission which has a long lifetime due to a spin forbidden radiative transition.1 The ms emission channel can therefore be assigned to emissive Mn centres in Tris SbMnCl.
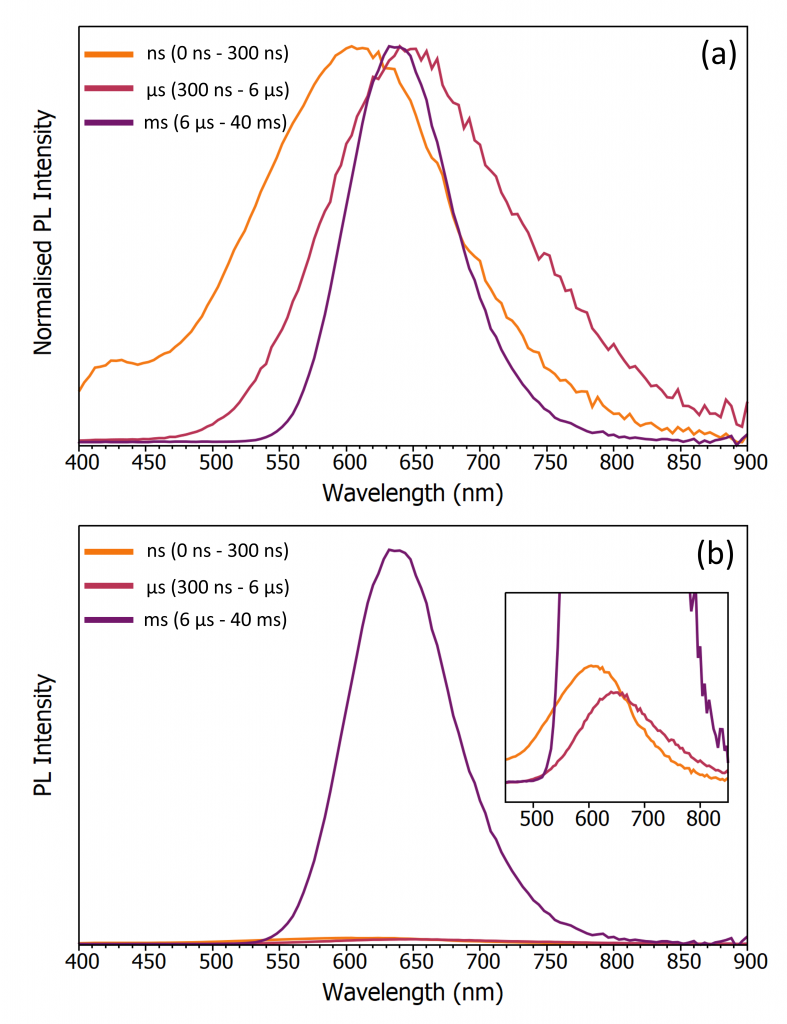
Figure 4: Emission spectra of the ns, µs & ms channels of Tris SbMnCl with (a) normalised intensities and (b) relative intensities.
The relative intensity of the three emission channels are compared in Figure 4b. The Mn centre emission is clearly stronger than the ns and µs channels of the Sb centres, with the Mn centre emission accounting for >95% of the total photoluminescence. Absorption and photoluminescence excitation studies have shown that at the 310 nm excitation wavelength used here, Sb centres are the dominant absorbing species and Tris MnCl is much less emissive than Tris SbMnCl when excited at this wavelength.1 This information in combination with calculation of >95% photoluminescence intensity from Mn centres obtained from the TRES reveals that the Sb centres are acting as antennae, harvesting the excitation energy and then transferring this energy to the emissive Mn centres that act as acceptors. Full details on the supporting measurements for this energy transfer mechanism can be found in the accompanying publication.1
Time-resolved emission spectroscopy (TRES) is a powerful technique for characterising the emission in materials with multiple emission channels. The FLS1000 can undertake automated TRES measurements in both TCSPC and MCS mode, covering the full range of lifetimes from picoseconds to seconds. A wide range of excitation sources are available to suit sample requirements including flashlamps, pulsed diode lasers, pulsed light-emitting diodes, supercontinuum lasers all of which can be used for TRES.