Menu
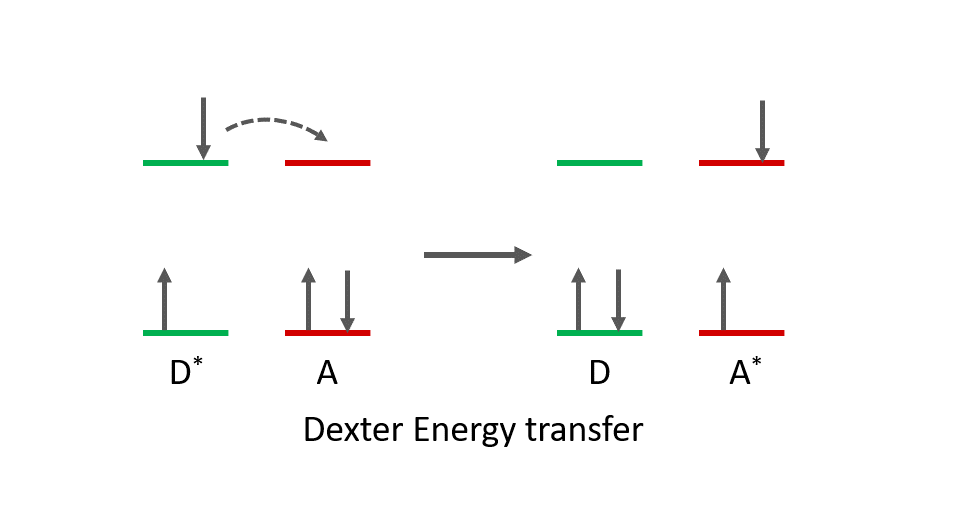
Dexter energy transfer, also called electron exchange energy transfer, is a photophysical mechanism where a simultaneous exchange of electrons occurs between donor and acceptor molecules. In Dexter energy transfer, an electron is transferred from the HSOMO (Highest Singly Occupied Molecular Orbital) of the donor in its excited state to the LUMO (Lowest Unoccupied Molecular Orbital) of the acceptor in its ground state while an electron is transferred from the HOMO (Highest Occupied Molecular Orbital) of the acceptor to the LSOMO (Lowest Singly Occupied Molecular Orbital) of the donor (Figure 1). At the end of the process, the acceptor molecule becomes excited, and the donor is deactivated. In contrast to FRET, which is singlet only process, in Dexter energy transfer both singlet and triplet energy transfer can occur.
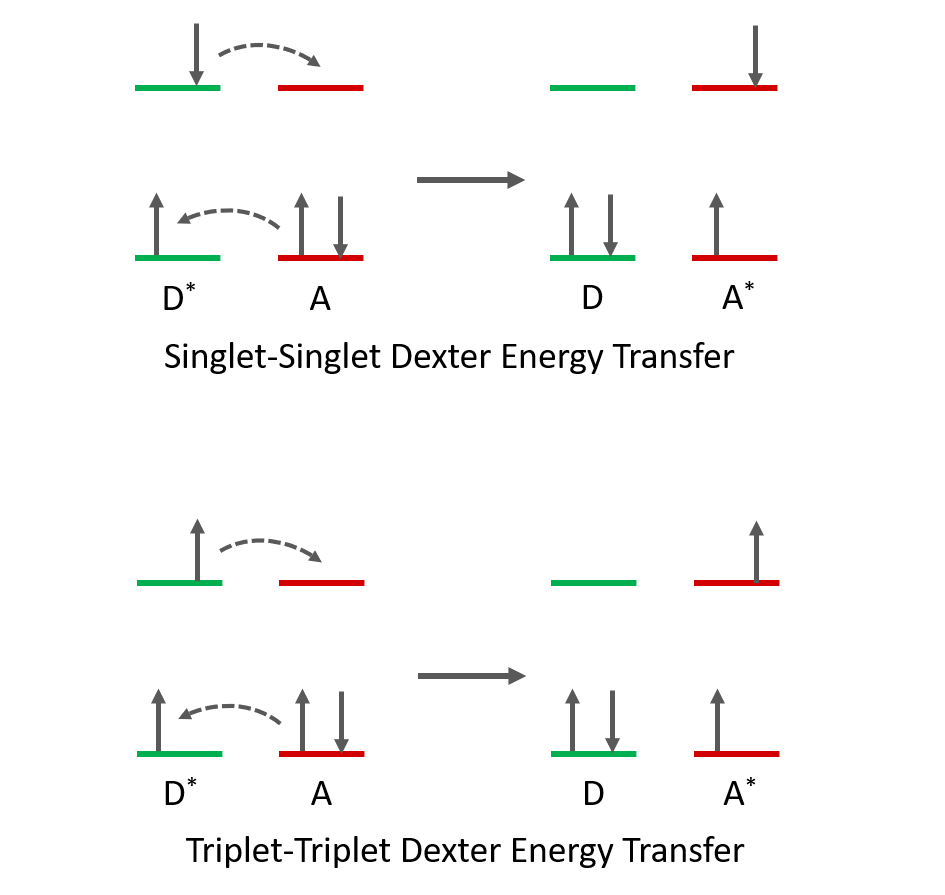
Figure 1. Dexter energy transfer mechanism between a donor (D) and acceptor (A). Excited state molecules are represented by *.
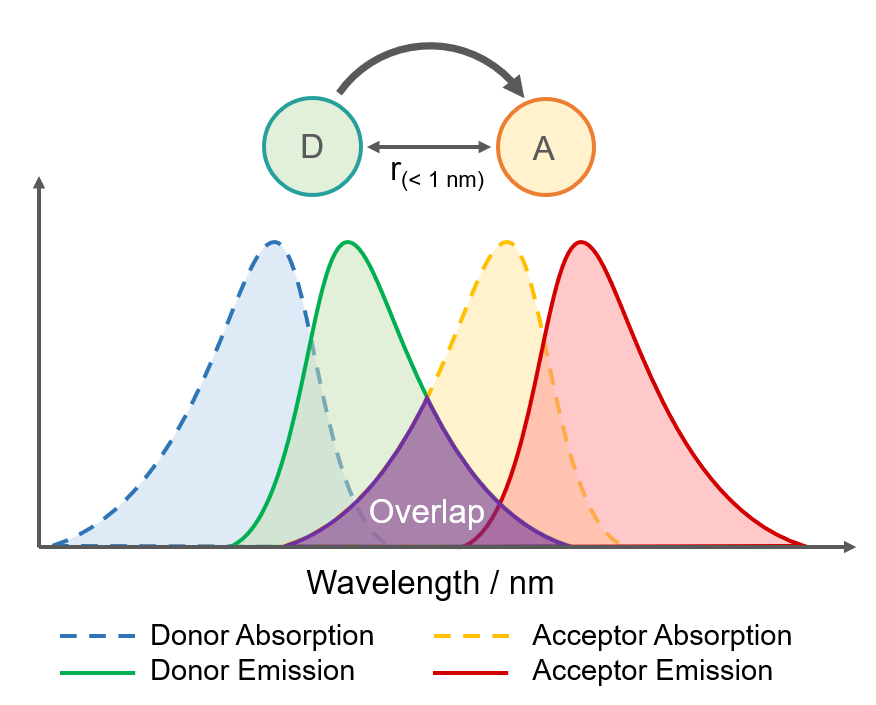
Figure 2. Requirements for Dexter Energy Transfer.
The Dexter energy transfer rate constant, kDET, is given by Eq. 1:

where K is an experimental factor related to the orbital interactions, r is the distance between the donor and acceptor, and L is the sum of Van der Waals radii of the donor and acceptor.
An important difference between Dexter and FRET energy transfer is their dependence on distance (Figure 3). Dexter energy transfer proceeds via an electron exchange mechanism that requires orbital wavefunction orbital and the transfer efficiency therefore falls off more rapidly (exponentially) compared with the dipole-dipole (1/r6) interaction in FRET. Dexter therefore occurs over shorter distances than FRET. Another important difference is that FRET is a singlet-only process while Dexter can proceed via both singlet and triplet excited states.
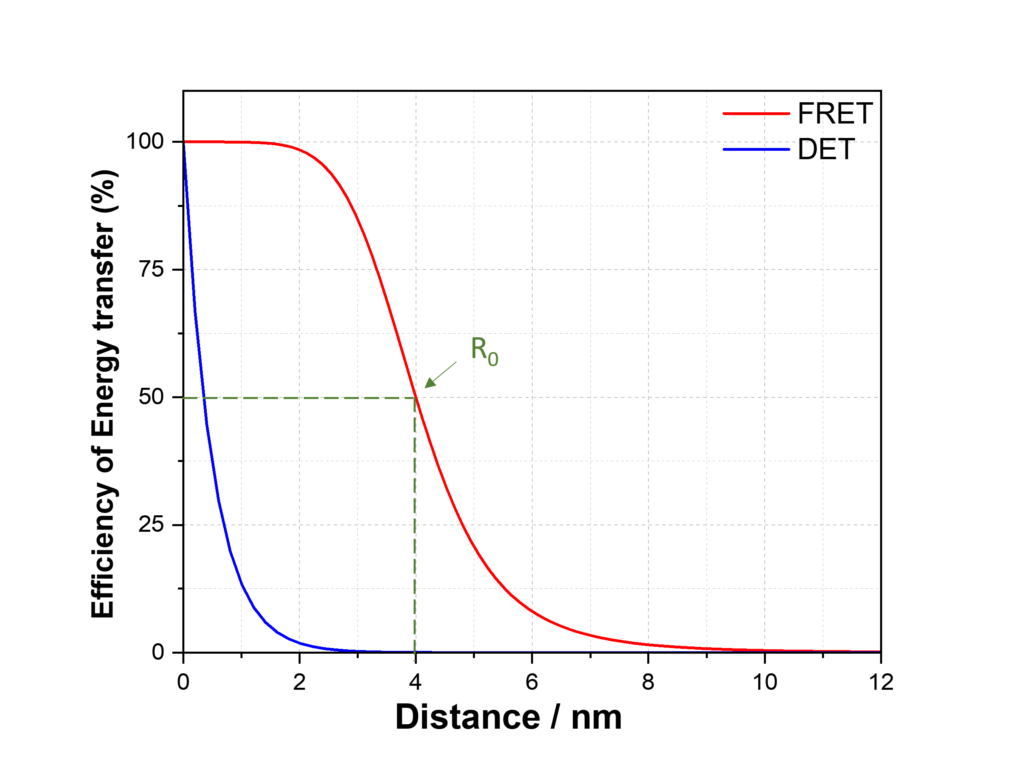
Figure 3: FRET and Dexter energy transfer dependence on distance. R0 is the Förster radius.
No results found.
No results found.