Welcome to Edinburgh Instruments’ blog celebrating our work in Raman, Photoluminescence, and Fluorescence Lifetime Imaging. Every month, we aim to highlight our pick for Map of the Month to show how our Raman and fluorescence spectrometers can be used to reveal all the hidden secrets in your samples.
In situ and operando Raman microscopy has become an essential tool for studying electrochemical processes and characterising constituent materials in batteries, fuel cells, and electrolysers. The technique can be used to determine the chemical composition of electrodes, electrolytes, and ion exchange membranes, which is invaluable because it allows for an understanding of the chemistry underpinning the overall electrical performance of a system to be gained. The experimental setup to perform electrochemical in situ or operando Raman microscopy requires the following components:
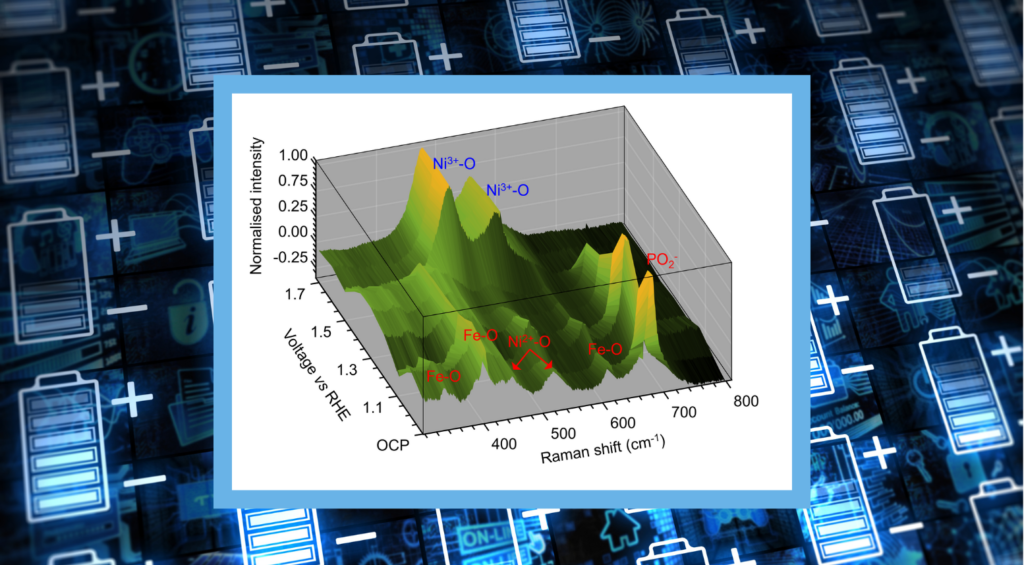
In this Map of the Month blog, we revisit our recent Research Highlight, Operando Raman Microscopy of a Water-Splitting Electrocatalyst, in which we reviewed a paper from the group of Professor Jingshan Luo at the Solar Energy Research Centre at Nankai University. In it, the RMS1000 Confocal Multimodal Microscope was used for the operando characterisation of a NiFePx water-splitting electrocatalyst. By coupling the RMS1000 with a three-electrode cell (with the catalyst operating as the working anode) and measuring the Raman spectra of their catalyst with variable potential, they were able to determine changes in the catalytic active sites that explained the performance of the catalyst during the oxygen evolution reaction.
The image above shows a map of the changing Raman signatures with increasing potential. Between open circuit potential (OCP) and 1.4 V, the spectra recorded from the electrode show signatures that indicate the presence of Ni2+, P, and Fe through their surface bonds with oxygen (shown in red). However, at 1.5 V and above, these spectral signatures are diminished, and two new bands appeared, which were attributed to Ni3+-O bonds (shown in blue). This indicated that the NiFePx was unstable and being converted to NiFe-layered double hydroxide (LDH) and provided a mechanistic explanation for why NiFePx did not outperform NiFe-LDH for oxygen evolution reaction efficiency.
If you are interested in finding out more about this research and how the Edinburgh Instruments RMS1000 and RM5 Microscopes can be used for electrochemical research, please check out our Research Highlight, Operando Raman Microscopy of a Water-Splitting Electrocatalyst, and the original article, published in the Royal Society of Chemistry Journal of Materials Research A.
No results found.