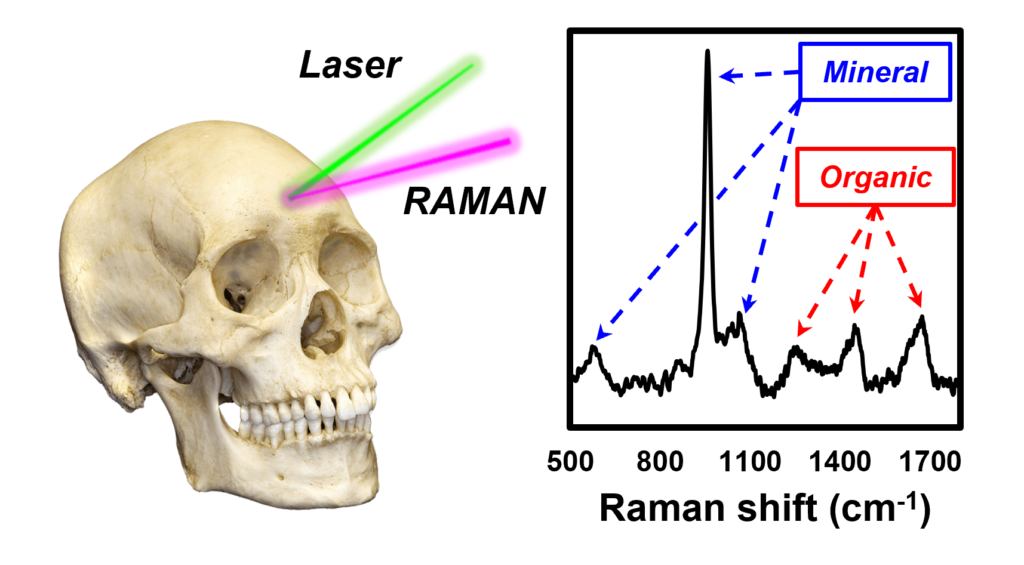
As well as rising from the grave to wander the earth and haunt us every Halloween, skeletons are made of hard, metabolically active bone tissues that perform several major functions to help our bodies thrive. These includes maintaining and supporting our shape, allowing us to have a dynamic range of movement, protecting our vital organs from damage, producing blood cells, and storing key nutrients.
Bone tissue consists of cells embedded within an extracellular matrix made up primarily of collagen and crystals of calcium hydroxyapatite. It is these organic and mineral constituents of the matrix that gives bone its strength. Despite being exceedingly sophisticated and refined, it turns out that there may be reason to sympathise with our spooky and supernatural skeletal chums. Bone tissue is susceptible to a wide range of diseases that have the potential to drastically diminish quality of (after)life, including osteoporosis and osteoarthritis. These disorders cause changes in the mechanical properties of bone that in turn can be understood by exploring its composition and structure.
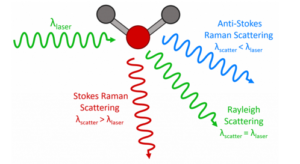
Raman spectroscopy is a terrific technique for studying biological tissues such as bone because it can be applied to fixed and fresh specimens with minimal sample preparation, it allows for non-destructive chemical analysis, and it provides us with a wealth of information in a single spectrum.1–3 In this blog post, we look at different types of bone samples using the Edinburgh Instruments RM5 Raman Microscope, Figure 1.

Figure 1. RM5 Raman Microscope.
The first sample analysed consisted of a segment of compact bone tissue fixed on a glass slide. Compact bone is extremely strong and dense in order to withstand bending and compression. It is found in the shaft of long bones such as the femur and the tibia. The slides were prepared specifically to show transverse and longitudinal sections of compact bone, Figure 2A and 2B. The spectrum from the transverse section of bone, excited with a 532 nm laser, is shown in Figure 2C. Like all biological samples, the tissue contains several diagnostic bands within the fingerprint region, which is typically between 400 cm-1 and 1800 cm-1, indicative of carbohydrates, DNA, lipids, and proteins. Additionally, bands associated with carbonate and phosphate anions from bone mineral are observed in the fingerprint region. However, their intensity is low because the sample was prepared specifically to show cells and therefore has a lower degree of mineralisation than untreated bone.
The high-wavenumber region of the Raman spectrum, Figure 2D and 2E, between 2700 cm-1 and 3500 cm-1 has bands indicative of C-H, N-H, and O-H stretching modes of biological species such as DNA, lipids, and proteins.4
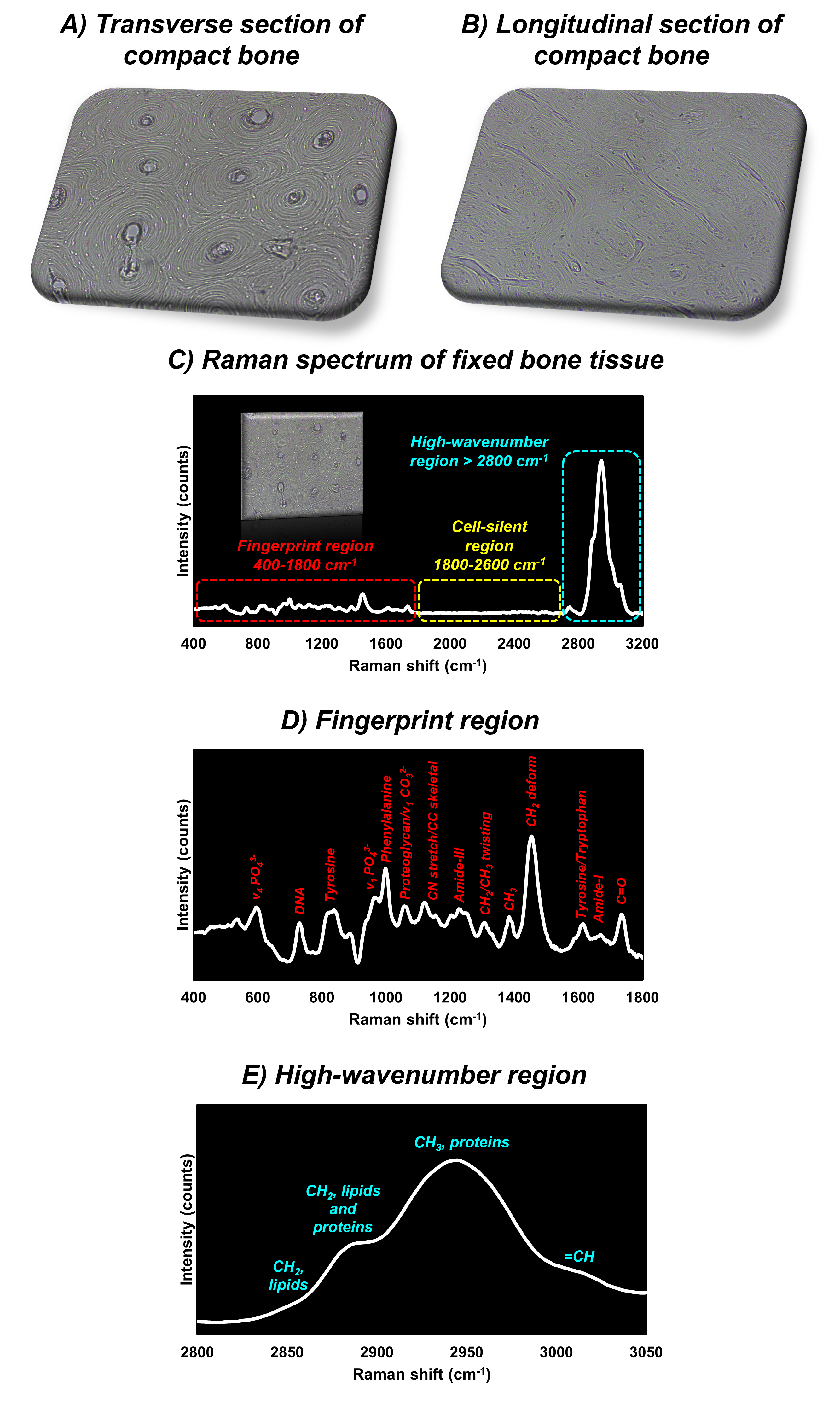
Figure 2. Raman spectroscopic analysis of fixed compact bone tissue.
The second kind of sample analysed, selected because it offers a great comparison to fully mineralised bone, was an untreated tooth. In this experiment, the Raman spectrum of the root of the tooth was collected under 532 nm excitation, which can be observed in Figure 3. The Raman spectrum of the untreated and mineralised tissue can be broadly categorised as before into fingerprint, cell-silent, and high-wavenumber regions. In Figure 3B we can now see that the bands suggestive of mineral components, such as carbonate and phosphates, are much more intense than the bands from the fixed compact bone tissue analysed before. This is best demonstrated by the most intense band at 960 cm-1, which can be assigned to the phosphate group found in the mineral component of the tooth and bone.
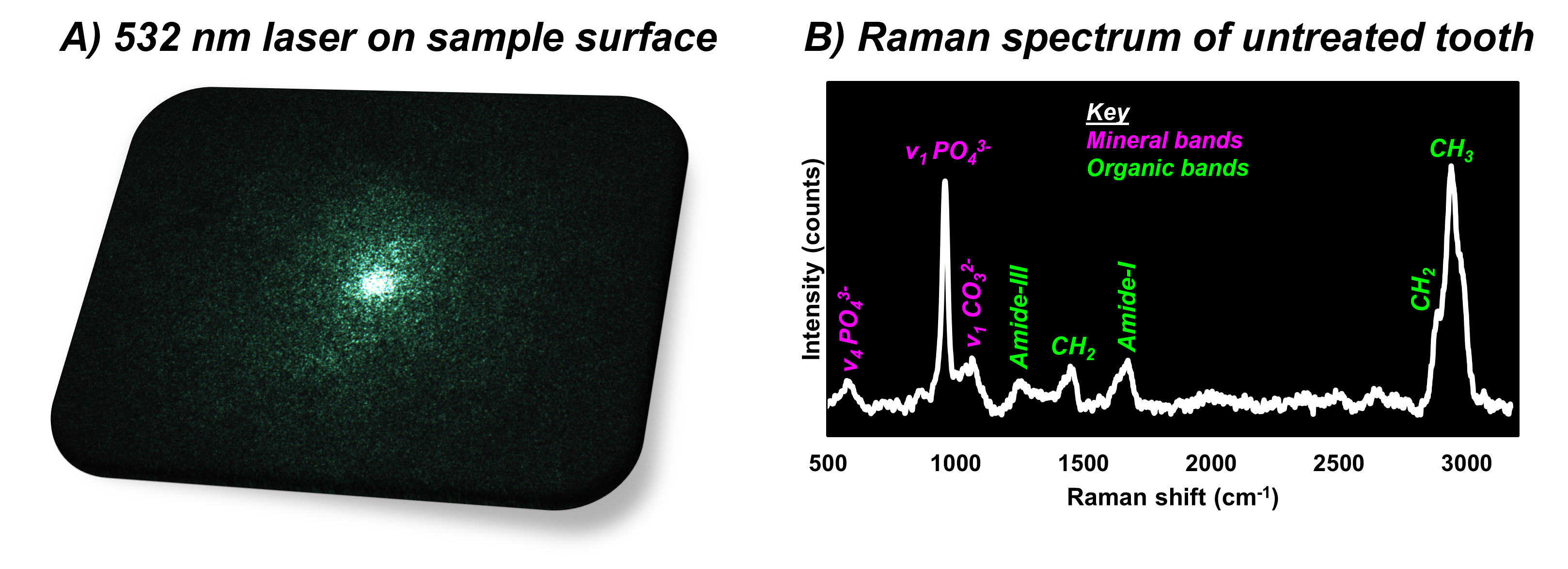
Figure 3. Raman spectroscopic analysis of an untreated tooth sample.
For more information on Raman spectroscopy, please visit our learning centre or check out our application notes.
If you have enjoyed this article, be sure to sign up to our eNewsletter or follow us on social media using the links below.