Welcome to our series, Science Explained. We know that science, and scientific concepts can sometimes be difficult. At Edinburgh Instruments, we aim to help! Science isn’t all formulas and complicated words only for old professors with crazy hair. Science should be accessible to everyone, and through our series, we hope to explain some scientific concepts in ways that everyone can understand. There might even be some experiments to try at home – but please be careful!
In our first instalment, which ties in with our 20 Years of Graphene series, we’re going to explain to you how graphene, diamond and graphite – 3 very different materials with their own unique properties can all be made of the same material – carbon!
Carbon is one of the most abundant elements known to us. It is vital for biological life and organic matter, and humans have harnessed different forms of carbon for energy for centuries.
Thanks to its electronic configuration, carbon can bond with up to four other atoms in various ways. Even on their own, carbon atoms can bond together in different structures. These different arrangements of carbon, known as allotropes, can have vastly different properties with uses in many different applications from batteries and jewellery to drug delivery.
In this blog, we will outline:

As we kick off our celebration of 20 Years of Graphene, the first allotrope we will discuss is graphene.
Graphene is a single layer carbon structure with a hexagonal arrangement. Despite being only one atom thick, graphene is one of the strongest materials known, yet is still lightweight and flexible.
This remarkable strength is just one of the outstanding properties of graphene that makes it a wonder material. Graphene also boasts excellent thermal and electrical conductivity.
In the two decades since its discovery, graphene has already found applications in industries such as medicine, electronics, energy, and sensors to name a few! Graphene and its applications are a huge area of interest both in academic and industrial research. We’ve only scratched the surface of its potential to improve the likes of renewable energy storage and targeted drug delivery.

Graphite is a naturally occurring mineral composed of crystalline carbon. Structurally, graphite is actually composed of many layers of graphene held together by weak Van der Waals forces. This is what gives graphite it’s softness.
As well as being soft, graphite also has electrical conductivity making it a useful material for batteries. Lithium-ion batteries are hugely important in day-to-day life, and due to graphite’s chemical stability it provides a stable host for lithium ions improving efficiency and reliability.
Other uses of graphite include in batteries and lubricants – its slippery layers, high temperature and chemical stability, and low shear strength make for a lubricant suitable for the most demanding of industrial settings.
Did you know at some point, you’ve probably used graphite? One of the most common uses of graphite is in pencils – the softness of the material makes it easy to use for writing.
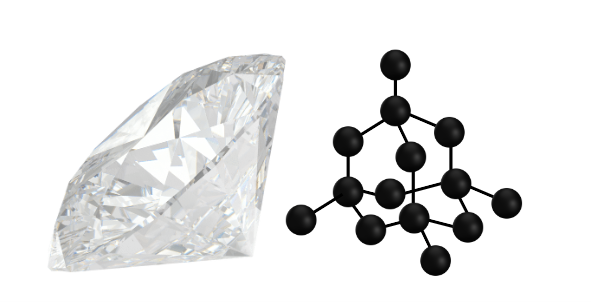
Often associated with jewellery and expensive taste, diamond is one of the most desired precious stones known, but on a molecular level diamond is just carbon.
Diamond serves as an excellent illustration of the significance of the carbon structure in each allotrope. Diamond is arranged in a rigid, tetrahedral covalently bonded network. This bonding obtains one of the hardest materials known, meanwhile in the pervious allotrope of carbon, graphite, the layers are bonded together by weak Van der Waals forces. This results in a soft material, but both are simply made of just one chemical element – carbon.
The hardness of diamond enables applications beyond its aesthetics. Industrial applications include oil rig drills and glass cutting. Diamond knives provide a much finer blade for smooth and precise cutting, so are now the “gold standard” for surgical tools.

Fullerenes are enclosed, or partially enclosed, hollow cage-like structures of carbon rings. They take the form of spheres, tubes, and other shapes.
The most famous fullerene is C60 known as buckminsterfullerene. This closed fullerene resembles a football so has its own nickname – a “buckyball”.
Fullerenes have high thermal stability, electrical conductivity, and the ability to form compounds with other elements and molecules.
Applications of fullerenes include drug delivery thanks to their cage like structure fullerenes can encapsulate or trap drug molecules and transport them for targeted delivery. Fullerenes bring some special electrical properties to the table which are highly customisable, i.e., their properties can be tweaked to improve our electronic gadgets. Fullerenes play a key role in making batteries more efficient, lightweight, and reliable, helping power everything from your smartphone to electric vehicles!

Similar to our first allotrope, graphene, carbon nanotubes are a single layer carbon atoms arranged hexagonally but rolled into a cylindrical tube shape, making them a type of fullerene.
Carbon nanotubes are incredibly strong and, like graphene, have electrical and thermal conductivity properties. They also have great flexibility and elasticity.
Carbon nanotubes have uses in a wide range of applications such as biomedical field where they are used in medical devices for early detection of diseases or in imaging techniques like MRI. Their extremely narrow tubular structure (we’re talking much thinner than a human hair) make them excellent at trapping tiny particles. This, combined with their high surface area makes them fantastic and efficient water filters. Carbon nanotubes, as mentioned, are incredibly strong whilst also remaining extremely lightweight. These properties have seen them used in sports equipment like tennis rackets, or even in airplanes to help make them stronger and lighter.
The allotropes of carbon highlight the significance that the structure of a molecule has on its properties. Although each of the materials highlighted contains only carbon atoms, their various structures result in vastly different properties, giving them their own unique properties that benefit different applications.
At Edinburgh Instruments, we have several application notes and blogs that highlight how our spectroscopic instruments can be used in the research and development of carbon allotropes:
Graphene
Graphene Oxide Photoluminescence
Carbon Nanotubes
Rapid Excitation Emission Matrix Analysis of Single Walled Carbon Nanotube

No results found.