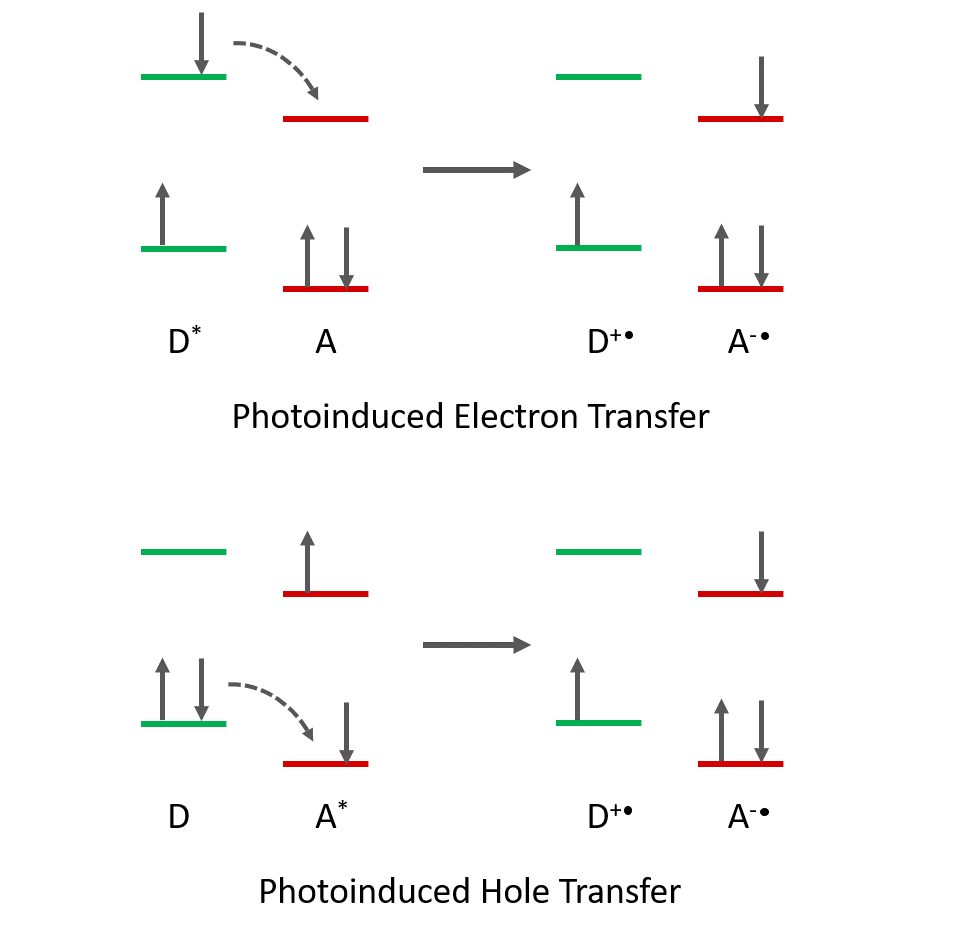
In photoinduced electron transfer, an electron is transferred from a photoexcited donor molecule to an acceptor molecule. The process results in charge separation and the formation of a radical cation of the donor and a radical anion of the acceptor (Figure 1). Transfer between a photoexcited acceptor to a ground state donor molecule is also possible but this is more commonly described as a photoinduced hole transfer from the photoexcited acceptor to the donor.
Figure 1. Photoinduced electron transfer and hole transfer mechanisms. Excited-state molecules are represented by *. The arrows show the direction of electron transfer for both electron transfer and hole transfer mechanisms.
Photoinduced electron transfer can occur through space or through bonds; however, this requires orbital overlap between D and A, therefore the distance between donor and acceptor is an important factor that influences the electron transfer rate constant. Intramolecular electron and hole transfer between donor and acceptor regions on the same molecule/polymer is also possible.
The energic driving force for charge can be calculated using the Rehm-Weller equation:
where ΔG is the free energy change for electron transfer. E(D+/D) and E(A/A–) are the reduction potentials of the donor and acceptor respectively and E00 is the energy required for photoexcitation from S0 to S1. The e2/εd term corrects for the electrostatic interaction between the charged donor and acceptor after charge transfer, e is the elementary charge (1.6 × 10-19 C); ԑ is the dielectric constant of the solvent; and d is the distance between charges.