In this Research Highlight, Raman microscopy is used to examine the structural changes occurring in a manganese-based organic-inorganic metal halide under high pressure. By combining a Raman microscope with a diamond anvil cell and adjusting the pressure of the sample, researchers were able to observe uniform octahedral compression of the crystal. This observation enhanced their understanding of the material’s pressure sensing capabilities.
Organic-inorganic hybrid metal halides (OIMHs) have emerged as promising materials for various applications, including photovoltaics, photoelectric detection, light-emitting diodes, and sensors. Their exceptional optical properties, which can be fine-tuned through structural manipulation, have garnered significant attention, particularly in pressure-induced enhancement and piezochromism.
Hydrostatic compression of OIMHs results in lattice distortion, leading to decreased interatomic distances and bandgap variation. This causes compression-induced changes in emission wavelength and intensity, meaning the material can be used for pressure sensing. Due to its high sensitivity to perturbations in interatomic distances, Raman microscopy is a powerful technique for studying pressure-induced changes in crystalline materials (Figure 1). It also allows high spatial-resolution interrogation of single crystals within pressure chambers such as diamond anvil cells (DACs).
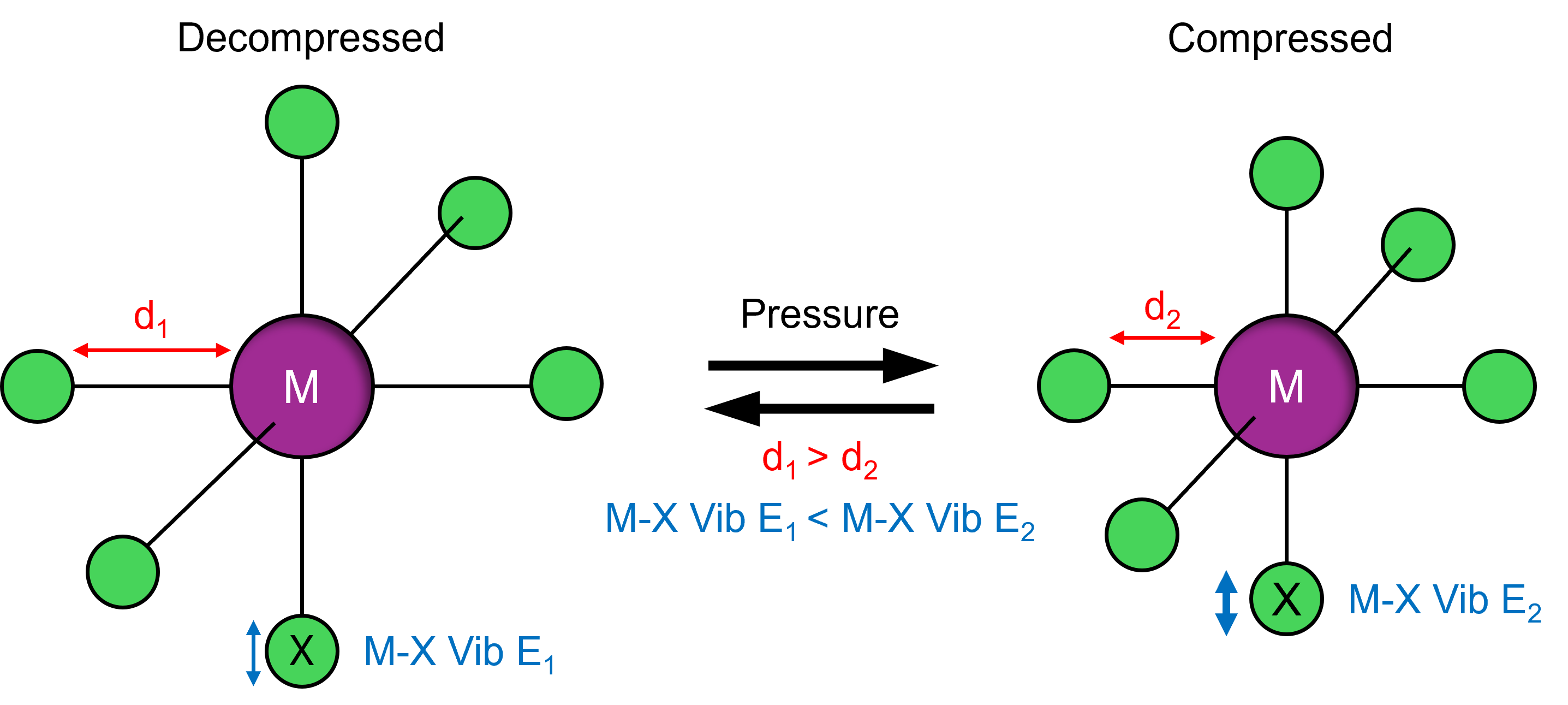
Figure 1. The effect of pressure on interatomic distance and vibrational energy.
This Research Highlight presents a recent study published in Chemistry of Materials by researchers at Nankai University in Tianjin, China.1 In this work, they used an Edinburgh Instruments RMS1000 Confocal Multimodal Microscope to investigate the pressure-induced piezochromism of an Mn-based OIMH.
A single crystal of zero–dimensional (0D) (TDMA)MnBr3(H2O)2 [TDMA = (tetrahydrofuran-3-yl) methylamine] was prepared for high-pressure Raman microscopy by placing it in a DAC, as depicted in Figure 2. The DAC comprised a pre-indented stainless-steel gasket sandwiched between two diamonds. Silicone oil was used as the pressure-transmitting medium, while a ruby crystal served for calibration. The DAC containing the sample was placed on the motorised stage of the RMS1000 for analysis.
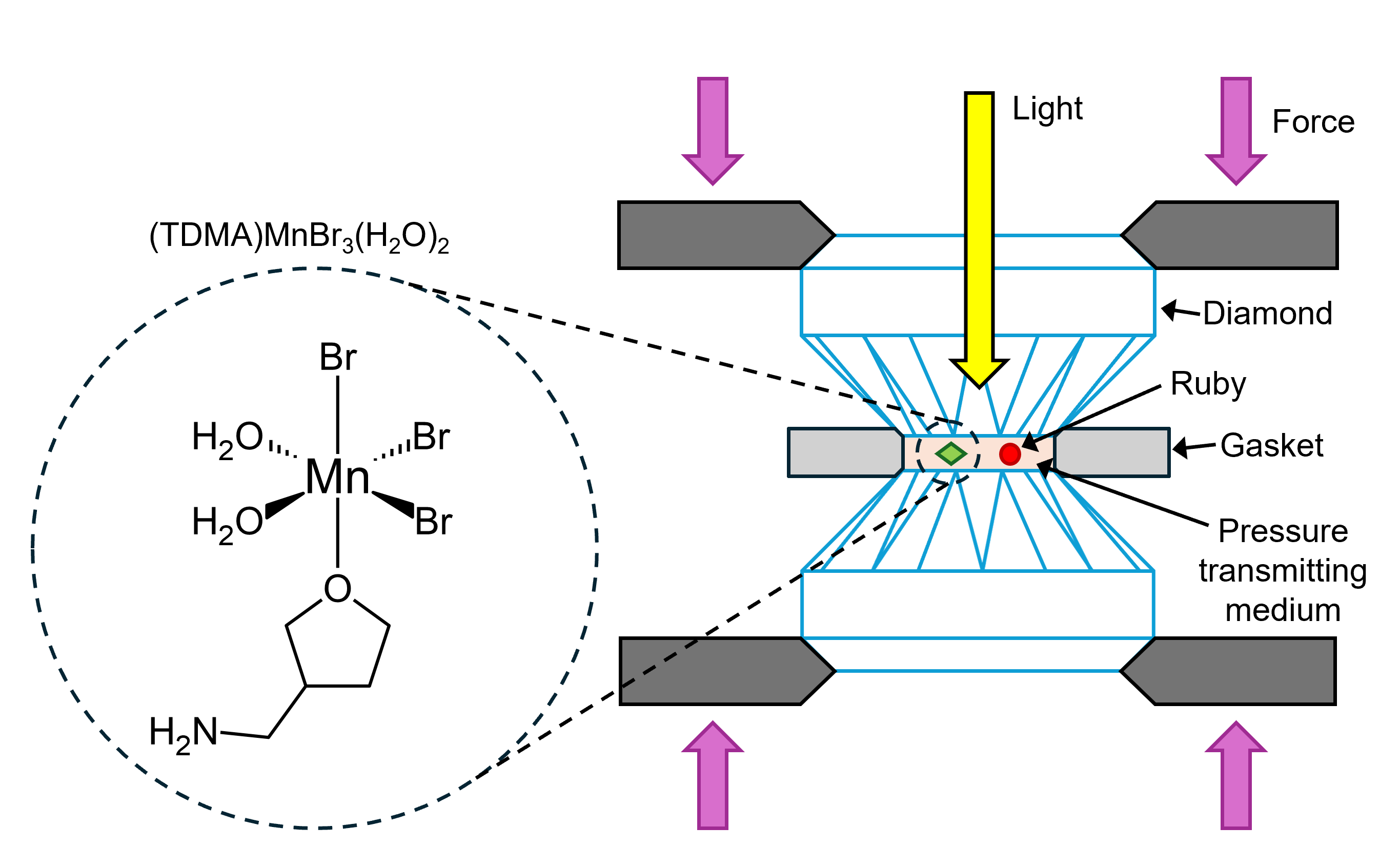
Figure 2. (TDMA)MnBr3(H2O)2 pressurised in a DAC.
High pressure Raman spectra of the crystal were recorded in the DAC using an RMS1000 equipped with a 785 nm laser, Figure 3. The spectra were recorded at various pressures between 1 atm and 11.4 atm.

Figure 3. Edinburgh Instruments RMS1000 Confocal Multimodal Microscope.
To explain why the crystal showed pressure-emission wavelength linearity under compression and decompression, (shown in the article,) the sample was analysed using high pressure Raman spectroscopy.
Figure 4 shows the Raman spectra acquired from the crystal with increasing pressure. The panel in Figure 4a shows the vibrational modes between 100 cm-1 and 400 cm-1 corresponding to Mn-X (X = Br and O), and Figure 4b displays modes between 600 cm-1 and 1200 cm-1 corresponding to the TDMA ligand.
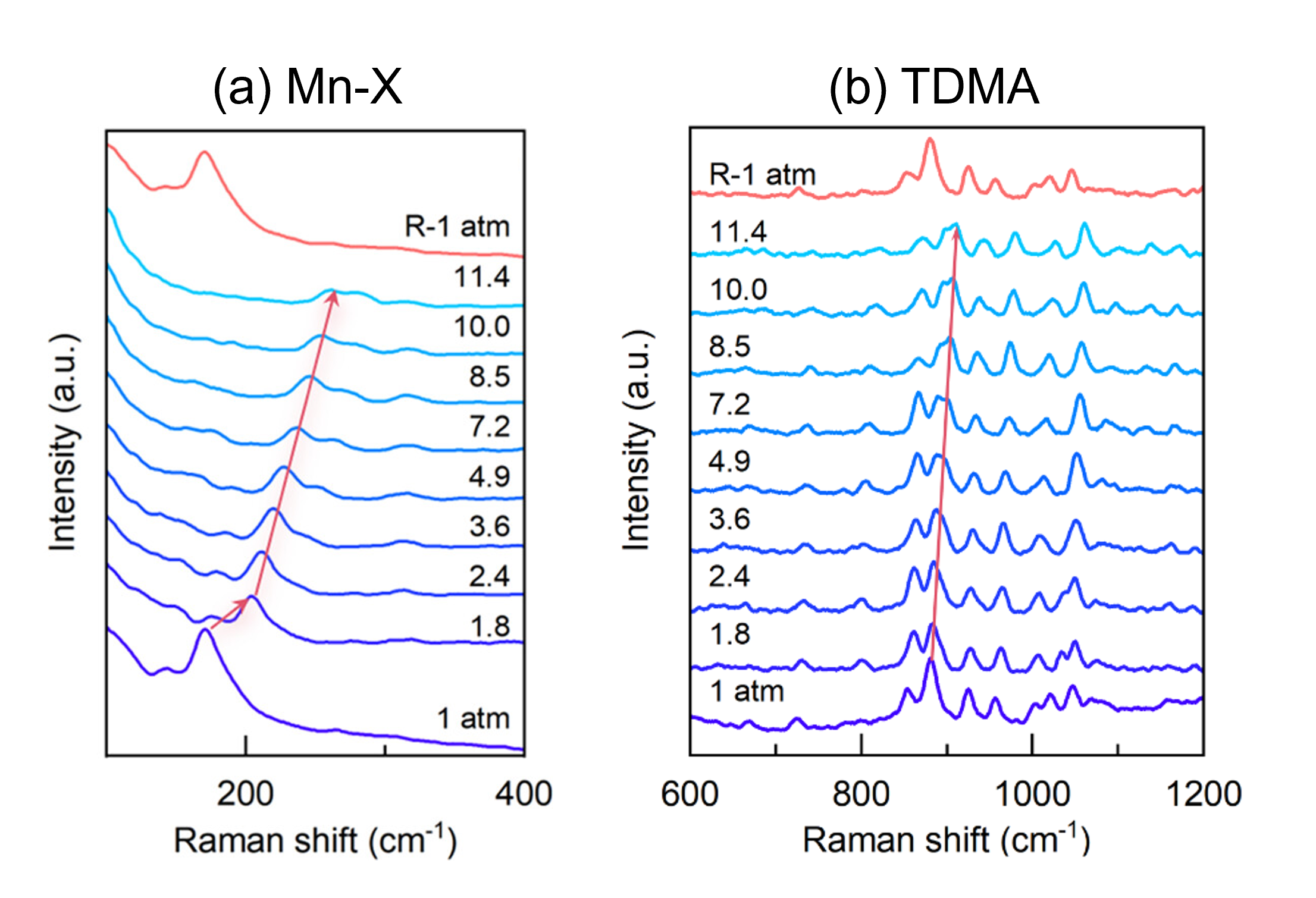
Figure 4. Raman spectra of (TDMA))MnBr3(H2O)2 under varying pressure. The image is reprinted from Zhang et al.1, Copyright (2024), with permission from American Chemical Society.
It can be seen in both plots that as the pressure increases, the position of all of the vibrational modes on the Raman shift axis also increases. This phenomenon is reversed when the pressure is released. This suggests that the interatomic distances within the octahedral lattice are decreasing; in other words, the octahedral complex is compressing under high pressure. The compression of the octahedra leads to an enhancement of the crystal field splitting of Mn2+, resulting in a decrease in bandgap and a redshift in photoluminescence (shown in the article). The Raman data combined with the pressure-emission wavelength linearity relationship observed indicates homogeneous compression of the octahedral complex rather than a phase transition. The stability of the sample under compression was explained by the large degree of intermolecular hydrogen bonding. Furthermore, the more pronounced peak broadening in the Mn-X region of the Raman spectra was assigned to a larger compression capacity of the [MnBr3(H2O)2]– relative to TDMA+.
This Research Highlight shows how an RMS1000 equipped with a DAC can be used to study materials under high pressure. The researchers in the study found a linear relationship between pressure and emission wavelength in (TDMA)MnBr3(H2O)2, indicating the potential of the material for pressure sensing. Raman spectral analysis in a DAC revealed homogeneous compression of the octahedral complex, which helped explain the observed piezochromic effect.
The results shown in this Research Highlight were published in Chemistry of Materials. More details can be found here: https://pubs.acs.org/doi/10.1021/acs.chemmater.4c00305?ref=pdf.


