The two techniques collect information about how molecular bonds vibrate when interacting with light. In IR spectroscopy, infrared light is absorbed by the molecule, and the molecule is excited directly to a higher vibrational energy level. Whereas in Raman spectroscopy, the molecule is irradiated and excited to a virtual energy state, it then relaxes to a different vibrational state, emitting a photon at a shifted wavelength from the incident photon, Figure 1.
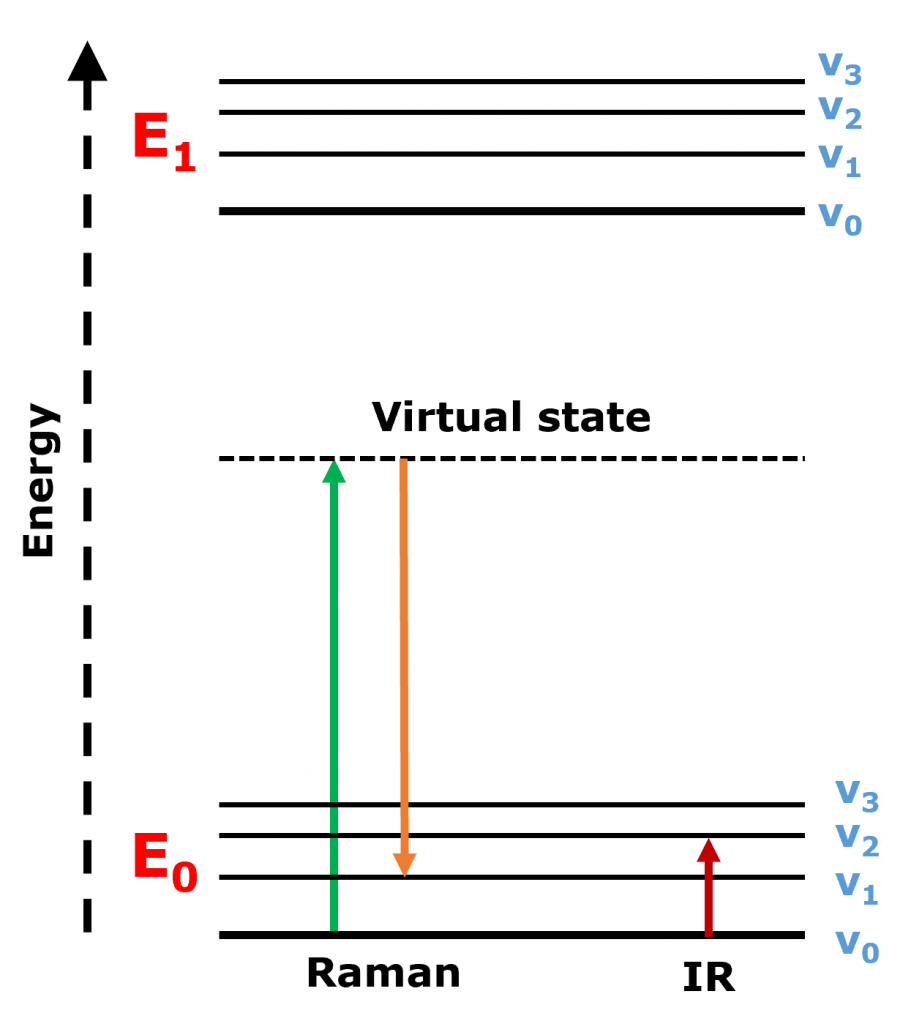 Figure 1. Jablonski diagram showing the energy transitions in Raman and IR spectroscopy.
Figure 1. Jablonski diagram showing the energy transitions in Raman and IR spectroscopy.
What is dipole moment? The dipole moment of a molecule refers to the separation of positive and negative charges within the molecule. A molecule with a non-zero dipole moment has an asymmetric charge distribution, resulting in a permanent dipole. The vibration causing a change in the dipole moment makes a specific mode IR active.
What is polarisability? Polarisability refers to the ability of a molecule’s electron cloud to be distorted by an external electric field. When a molecule is subjected to an incident photon, the electric field associated with the photon induces an oscillation in the molecule’s electron cloud. When the molecule undergoes a vibrational or rotational transition, the distribution of electron density within the molecule changes, resulting in a change in polarisability. The vibration causing a change in the polarisability makes a specific mode Raman active.
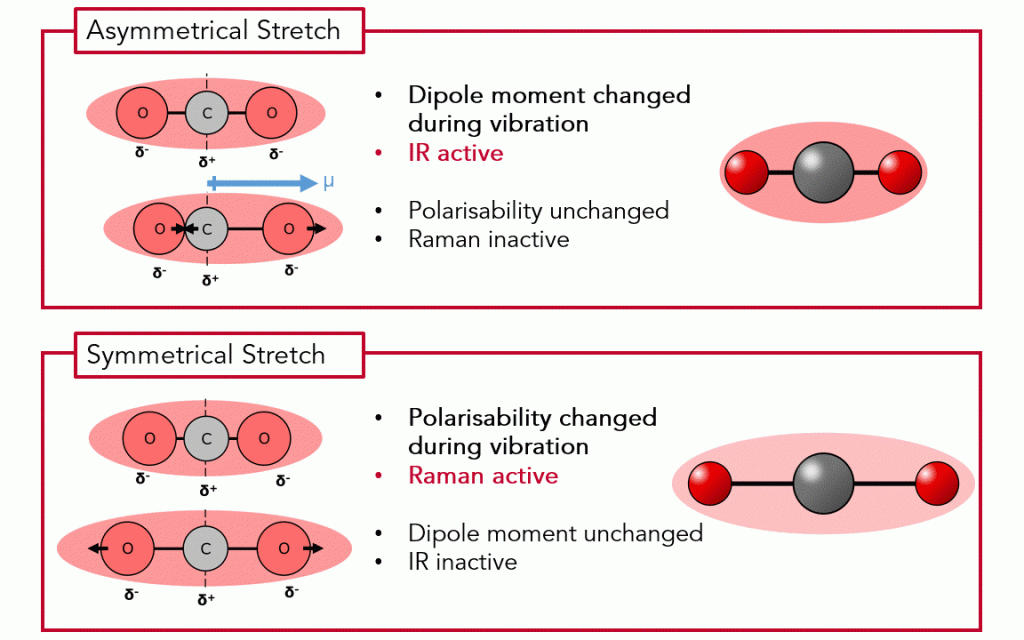
Figure 2. CO2 stretching vibrations for IR active vibrations (left) and Raman active vibrations (right)
CO2 has 3 atoms, and it is a linear molecule, therefore, there are 3N-5 = 4 vibrational modes. Figure 2 shows the two of these vibrations, termed asymmetric and symmetric stretching, respectively.
There is another form of vibration termed bending, here the bond angle changes during the vibration. There are four types of bending possible: wagging, twisting, scissoring, and rocking. An IR or Raman spectrum will be composed of bands caused by both stretching and bending vibrations. CO2 also has two bending vibrations; they have the same frequency and only differ in the direction they move, making them degenerate. These vibrations are IR active but since they are degenerate, they produce one peak in the IR spectrum of CO2.
An advantage of Raman spectroscopy is when investigating samples containing water. H2O is a very weak Raman scatterer meaning there is no influence on the Raman spectrum from water. In contrast, hydroxyl groups absorb IR radiation strongly and can cause substantial issues when trying to analyse aqueous samples with IR spectroscopy.
Whilst Raman spectroscopy does not suffer from water interference, it can suffer significantly from fluorescence interference. Raman scattering is an inherently weak phenomenon, fluorescence is orders of magnitude stronger and can be induced in the sample, substrate, or optical elements from the Raman laser which can then obscure Raman bands. IR radiation will not induce fluorescence because it does not allow for electronic excitation.
Sensitivity is always important when analysing samples, and of the two technique IR spectroscopy is generally more sensitive. However, both offer significant individual advantages in terms of sensitivity to specific functional groups. For example, Raman spectroscopy is especially sensitive to lattice vibrations in crystals for studying polymorphism, whilst IR spectroscopy is particularly sensitive to studying reaction intermediates in low concentrations.
Practically, an IR spectrometer is a simpler instrument. Raman measurements often require some testing to ascertain the best parameters for a sample, such as excitation source and grating selection. This is why IR spectrometers are commonly used in undergraduate chemistry laboratories whilst Raman spectrometers tend to be used in research settings. However, the advances of both instruments and their components over the past 20 years have increased the popularity and ease of use of the techniques.
Raman and IR spectroscopy are both powerful techniques and offer rapid and non-destructive analysis in all application areas. Whilst there are some general recommendations that can be made over which technique to pick, in reality, samples are complex, and both can offer advantages. Their different selection rules make them truly complimentary techniques and when used together they provide full sample characterisation.

No results found.