One particularly abundant green energy source, which is a promising alternative to fossil fuels, is hydrogen. Unlike methane, which produces carbon dioxide when burned, the only by-product from hydrogen combustion is water. One of the most common methods used to produce hydrogen is electrolysis, which uses electricity to split water into hydrogen and oxygen. Electrolysis can result in zero greenhouse gas emissions, depending on the source of electricity used. Among the various methods for designing water-splitting electrolysers, anion-exchange membrane water electrolysis (AEMWE) is particularly promising.
AEMWE uses a semipermeable anion-exchange membrane that conducts OH– ions, separating the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) chambers and providing insulation between the anode and cathode, Figure 1. Compared to other methods, AEMWE allows for a more compact electrolyser design, lower ohmic losses, higher gas purity, improved operational safety and facilitates the use of low-cost transition metal-based electrocatalysts that function at the electrode surface or can be the electrode itself. This method is a significant advancement in the field, and its optimisation is a crucial avenue of research for allowing the up-scaling of AEMWE electrolysers into commercial use.
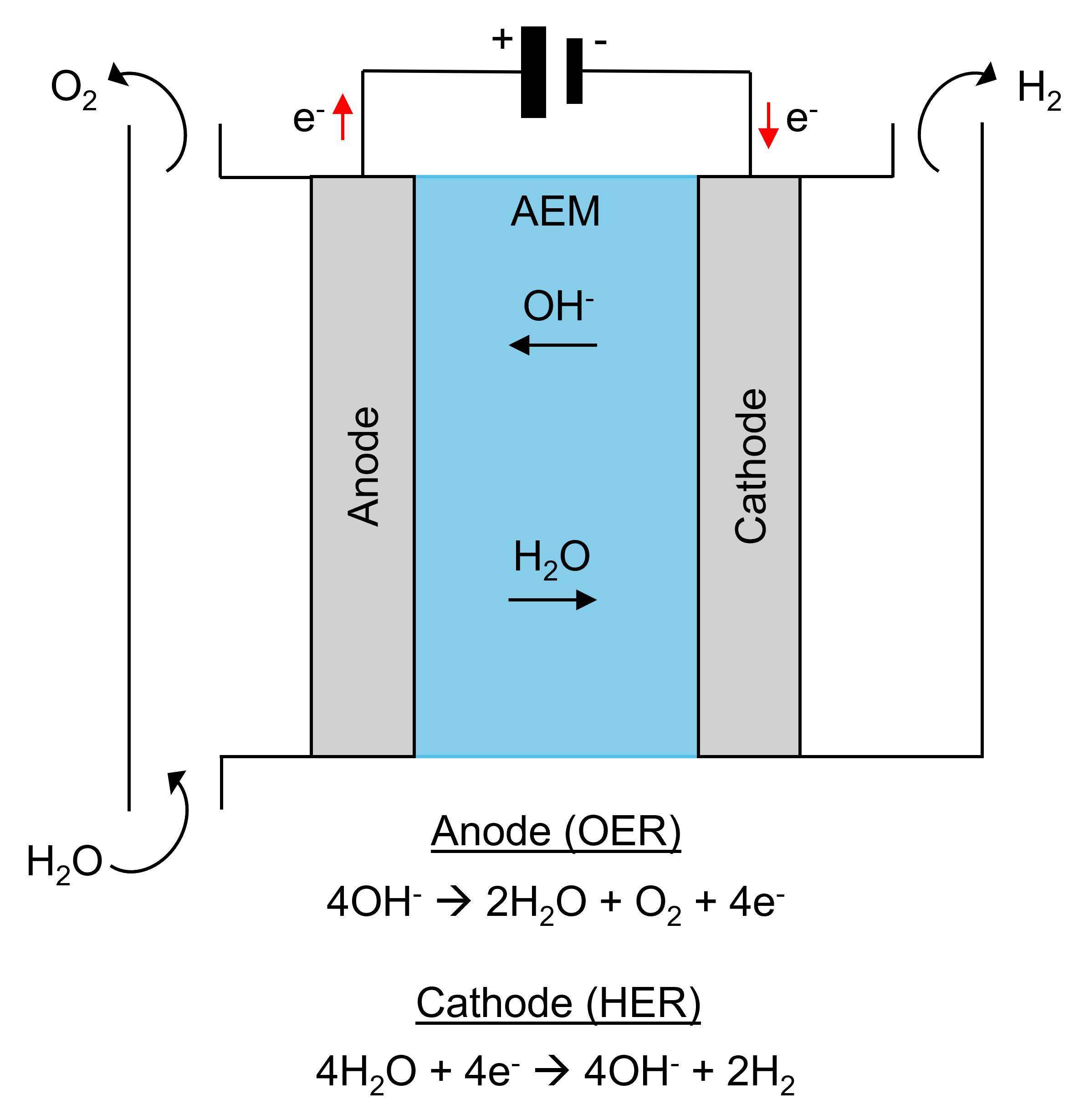
Figure 1. Principle of anion-exchange membrane water electrolysis (AEMWE) for hydrogen production in alkaline solution.
Here, we summarise the results of our RMS1000 Confocal Microscope users at Nankai University, who recently published their work in the Journal of Materials Chemistry A. In this article, they reported the development of a NiFePx electrocatalyst that showed enhanced hydrogen evolution reaction (HER) performance in alkaline AEMWE electrolytes when compared with the more commonly used NiFe-layered double hydroxide (NiFe LDH). They used operando Raman microscopy to characterise the NiFePx and elucidate compositional changes occurring during an oxygen evolution reaction (OER). By coupling the RMS1000 to a working electrochemical cell, it was possible to gain mechanistic insights into the chemistry occurring at the catalyst surface and link these insights to electrochemical performance.
The composition of the NiFePxelectrocatalyst and mechanistic changes on the surface of the material during an OER were characterised using an Edinburgh Instruments RMS1000 Confocal Microscope, Figure 2. The system was equipped with a 532 nm laser, an 1800 gr/mm diffraction grating and a CCD camera. Spectra were recorded over 200 seconds (100 accumulations of 2 s acquisition time spectra).

Figure 2. Edinburgh Instruments RMS1000 Confocal Microscope.
The NiFePxelectrocatalyst was synthesised in a two-step process involving the hydrothermal growth of NiFe LDH on Ni foam, followed by conversion using a phosphidation process that involved exposing the intermediate to PH3. gas.
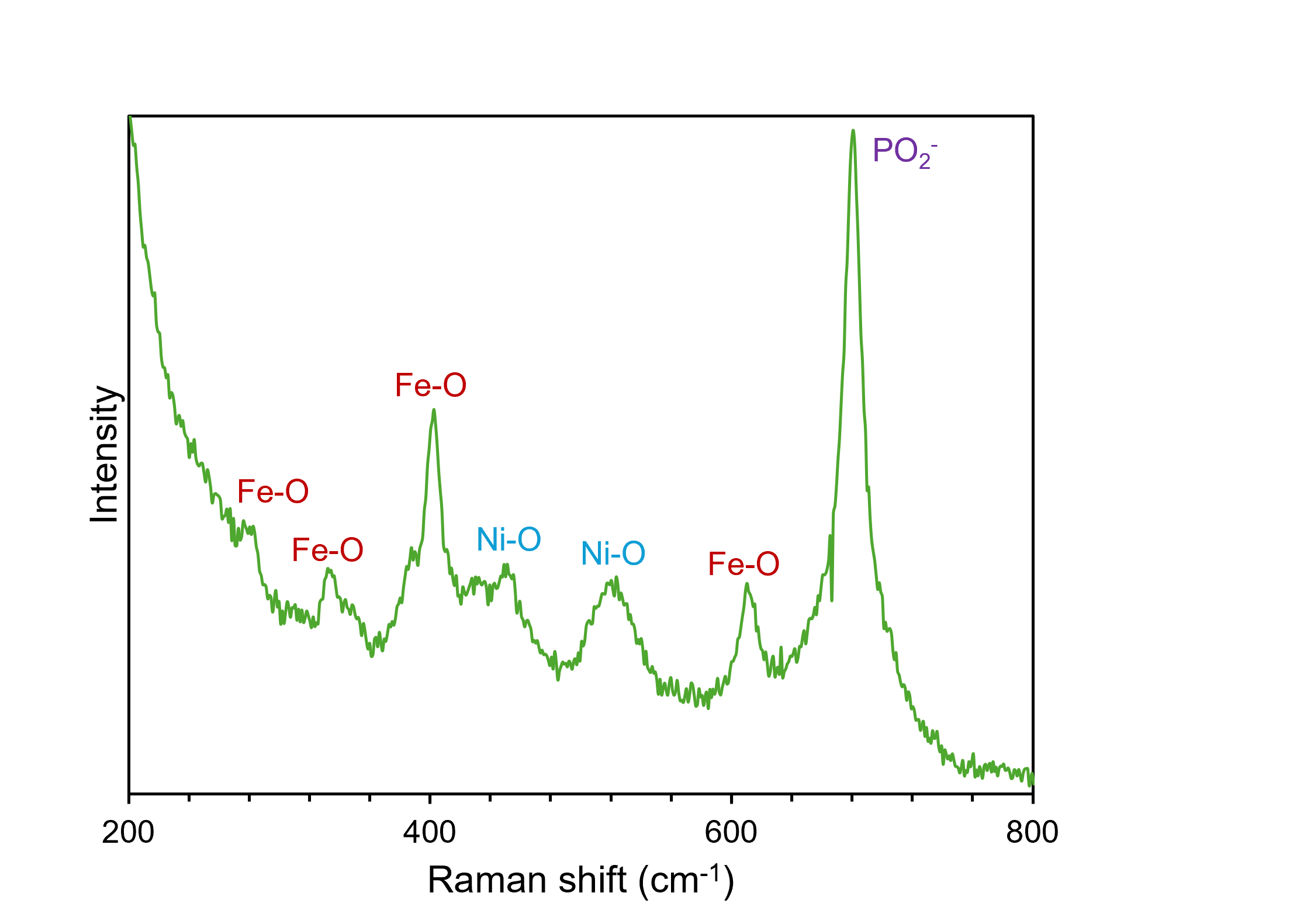
After synthesis, Raman microscopy was used to confirm the coexistence of Ni, Fe, and P in the electrocatalyst. The Raman spectrum of the material, shown in Figure 3, also indicates the presence of oxygen in the sample, which was attributed to incomplete conversion of the NiFe LDH or surface oxidation after it was exposed to air. The 532 nm laser is ideal for analysing samples that do not exhibit fluorescence because it allows for better spectral sensitivity than a higher wavelength visible laser, such as 638 nm and 785 nm. The 1800 gr/mm grating was used because it provides a high spectral resolution. The bands at 281 cm-1, 332 cm-1, 402 cm-1, and 610 cm-1 could all be assigned to vibrational modes of Fe2O3. Ni-O bonds were also deemed to be present on the surface of the catalyst because of the presence of bands at 453 cm-1 and 520 cm-1. These modes, in particular, were evidence of oxidation of the electrocatalyst surface. The prominent peak at 680 cm-1, indicative of symmetric stretching of PO2– groups, confirmed the presence of P in the material structure. The high level of spectral resolution and, hence, molecular specificity obtained when using Raman spectroscopy means that it is an excellent technique for characterising heterogeneous materials such as this.
 x using Raman spectroscopy” width=”600″ height=”430″ />
x using Raman spectroscopy” width=”600″ height=”430″ />
Figure 3. Compositional analysis of NiFePx using Raman spectroscopy.
Tests showed that the NiFePx catalyst had superior HER performance than the NiFe LDH catalyst, but both catalysts had similar OER performance. Operando Raman microscopy was used to determine the mechanistic reasons behind this observation. For this study, spectra were recorded of the NiFe LDH and NiFePx under controlled potential in a three-electrode cell consisting of the working electrode under investigation, a Pt plate counter electrode, and an Ag/AgCl reference electrode, Figure 4. Operando Raman microscopy is an excellent technique for gaining structural insights into samples that can be used to rationalise electrochemical performance. The study was performed in a solution of 1 M KOH. During the Raman study, the working electrode was scanned anodically from open circuit potential (OCP) to 1.7 V versus a reversible hydrogen electrode (RHE).
Figure 4. Schematic of the three-electrode cell used for operando Raman measurements.
Firstly, the NiFe LDH was installed as the working anode in the electrochemical cell and Raman spectra were recorded at different potentials. As the potential was increased, the bands corresponding to Ni2+-O species on the sample surface were diminished, and alternative bands at 476 cm-1 and 559 cm-1, assigned to Ni–3+-O, became prominent, Figure 5. These spectral changes with increasing potential reflect the oxidation of the electrocatalyst surface during OER and the formation of NiOOH species.
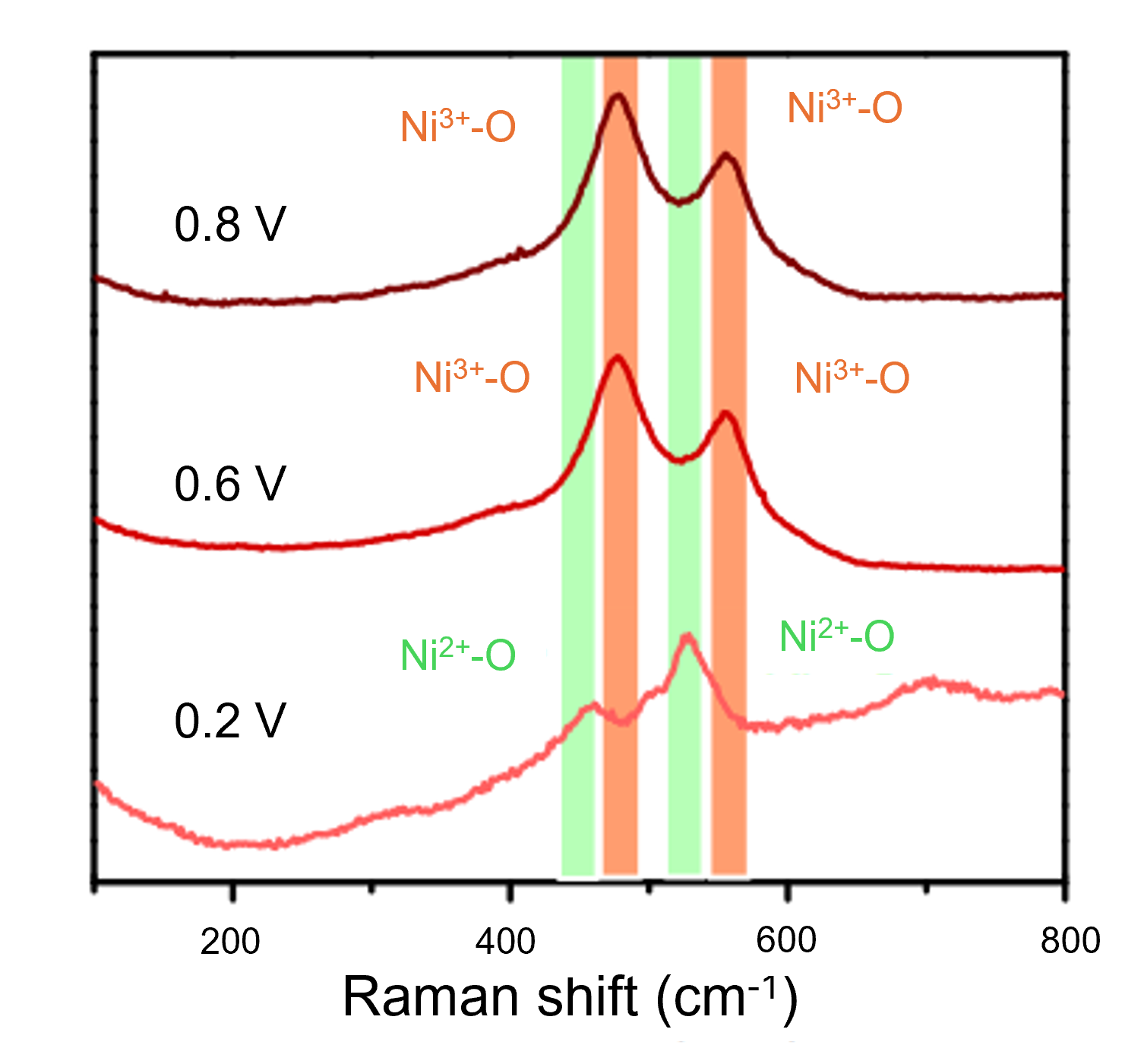
Figure 5. Operando Raman spectroscopy of NiFe LDH anode during an OER.
The same study was performed with NiFePx as the working anode, Figure 6. Between OCP and 1.4 V, the Raman spectra recorded from the anode show similar vibrational signatures to the spectrum in Figure 3, including those attributable to Fe-O, Ni-O, and PO2–. However, at 1.5 V, the band corresponding to PO2– dampens, the Ni2+-O bands diminish, and the Ni3+-O bands become prominent. These observations indicate that when used as an anode for the OER, NiFePx is unstable and is converted to NiFe oxide/hydroxide. This study provided mechanistic evidence that phosphides are unstable under electrochemical OER and explained why NiFePx did not outperform NiFe LDH in terms of OER efficiency.
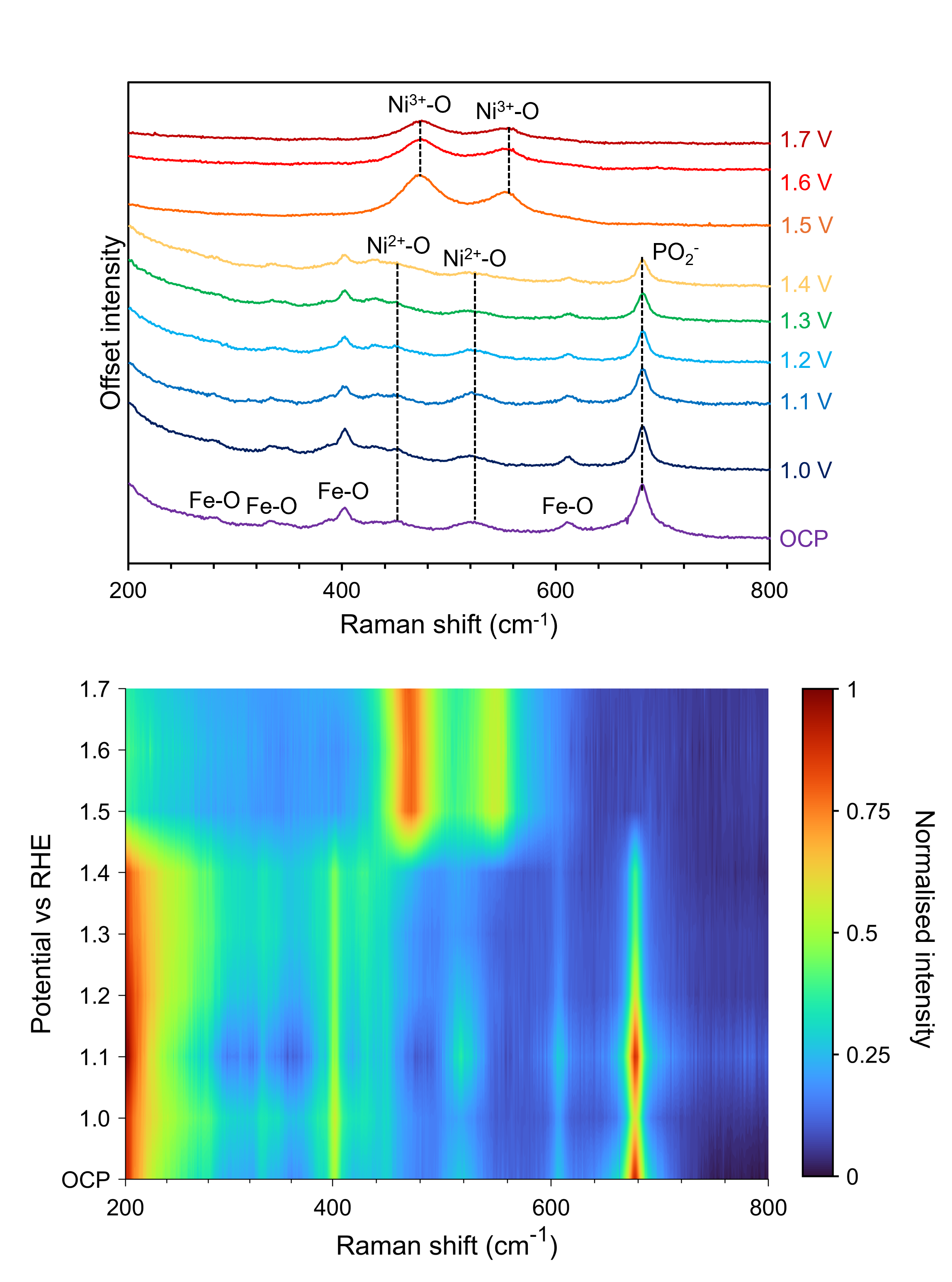
Figure 6. Operando Raman spectroscopy of NiFePx anode during an OER.
An Edinburgh Instruments RMS1000 Confocal Microscope was used to determine the composition of novel electrocatalysts for enhanced HER in water-splitting. By coupling the RMS1000 with a three-electrode cell and measuring operando Raman spectra of the catalyst, changes in the catalytic active sites could be determined, and electrochemical performance could be better understood.
The results shown in this Research Highlight were published in the Journal of Materials Chemistry A, and more details can be found here: Transforming NiFe layered double hydroxide into NiFePx for efficient alkaline water splitting.
We would like to thank the research group of Professor Jingshan Luo at the Solar Energy Research Centre at Nankai University for providing the data used in this Research Highlight.

