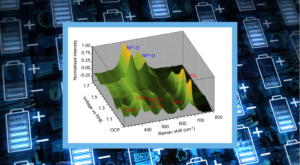
Potassium ion batteries (KIBs) are a promising, eco-friendly alternative to lithium-ion batteries. Potassium is more earth-abundant than lithium (2.09% versus 0.0017%), making it more cost-effective and less prone to supply chain issues. It is also a Group 1 alkali metal, which means it has chemical properties similar to lithium.
Furthermore, KIBs, Figure 1, can theoretically provide higher power densities than LIBs because of the lower standard reduction potential of K/K+ than Li/Li+ and faster K+ ion diffusion.1,2 However, KIB research is still in its infancy compared to the much larger and mature LIB industry, and significant development challenges must be overcome before the technology can be seen to compete.
One avenue of research is optimising the electrolyte, which heavily influences the formation and stability of the solid electrolyte interface and ion intercalation within the electrodes. This Research Highlight shows the recent work of a group at Northeastern University in Shenyang, China, who investigated the effect of electrolyte chemistry on the performance of the graphite anode in a KIB.3
They used the Edinburgh Instruments RM5 Raman Microscope to study the solvation mechanism of ions in different electrolyte formulations and rationalise the observed electrochemical performances in reconstructed cell experiments. Their work was published in the Chemical Engineering Journal.
Figure 1. Potassium-ion battery structure and operating principles.
Carbonate electrolytes were designed for use in a K0.5MnO2||graphite-based KIB. They consisted of an ethylene carbonate and diethyl carbonate (EC/DEC) solvent and different concentrations (1 M, 2 M, and 4 M) of a potassium bis(fluorosulfonyl)imide (KFSI) salt, Figure 2.
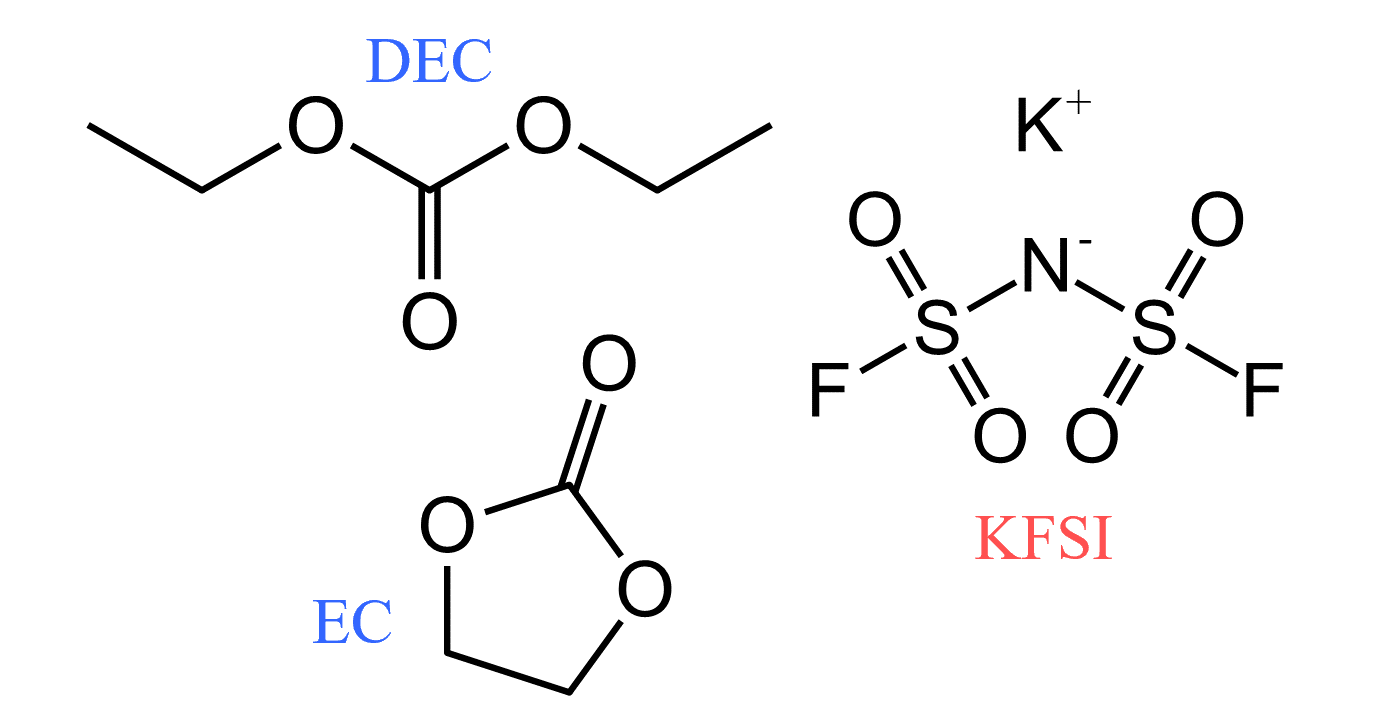
Figure 2. Chemical structures of solvent and salt used in the study.
The electrolytes were held within a glass capillary tube and placed under the objective lens of an RM5 Confocal Raman Microscope equipped with a 532 nm laser, Figure 3. The Raman spectrum of each sample was then recorded.

Figure 3. Edinburgh Instruments RM5 Confocal Raman Microscope.
Initial electrochemical tests were conducted on a graphite anode in the 1 M, 2 M, and 4 M KFSI EC/DEC electrolytes, and they indicated that the anodic rate and cycling performance were both enhanced when the concentration of the KFSI salt was increased. To understand this observation, the change in the interactions between solvent molecules and dissolved ions with increasing salt concentration in the electrolyte was investigated using Raman microscopy. This data is shown in Figure 2 of the original article.3
The Raman spectra of the S-N stretching vibration in the FSI- anion showed a shift to higher wavenumbers and broadening with increasing KFSI salt concentration in the EC/DEC electrolyte. This was attributed to the formation of ion pairs and aggregates, as evidenced by the increasing relative intensities of bands corresponding to these species. At 1 M KFSI, the FSI- anions are primarily free. At higher concentrations, contact ion pairs and larger aggregates form due to increased cation-anion interactions facilitated by the low dielectric constant of the solvent. The convolution of closely spaced bands from these species resulted in the observed shifts and broadening. These findings suggest that high-concentration electrolytes with low dielectric constant solvents, such as the 4 M KFSI electrolyte, may enhance the performance of graphite anodes in potassium-ion batteries.
An RM5 Confocal Raman Microscope was used to determine solvation mechanisms in different KIB electrolyte formulations. The Raman spectra provided information about the relative concentration of different solvation structures in the electrolytes, which could be correlated to the electrochemical performance of the graphite anode in a KIB.
The results discussed in this Research Highlight were published in the Chemical Engineering Journal. More details can be found here: https://www.sciencedirect.com/science/article/pii/S1385894724030274?via%3Dihub.
References