Since the discovery of graphene two decades ago, two-dimensional (2D) inorganic materials have been widely studied. Among these, one class consisting of 2D carbides and nitrides of transition metals has generated a surge of interest, with thousands of publications in the past decade since it was first reported in 2011.1 These materials, known as MXenes, are unique in their composition and properties.
MXenes have the general chemical formula Mn+1XnTx, comprising n + 1 atoms (n = 1-3) of one or more group 3-6 early transition metals (M) interleaved by n atoms of carbon and/or nitrogen (X). The surface termination groups (Tx), which can consist of -F, -O, -OH, or -Cl, on the exposed outer M layers, enhance the versatility and broaden the potential applications of the materials.2
MXenes are typically prepared by top-down etching of a MAX (Mn+1AXn) phase precursor, where A is a group 11-16 element such as Al, Si, or Ga, traditionally using hydrofluoric acid. The weaker M-A bonds are selectively broken during this process, and the uncoordinated M atoms are saturated by reacting with Tx groups from the etchant. The resulting MXene sheets can then be delaminated to yield individual layers of Mn+1XnTx. The etching process and the chemical structure of the most published MXene, titanium carbide (Ti3C2Tx), are shown in Figure 1.
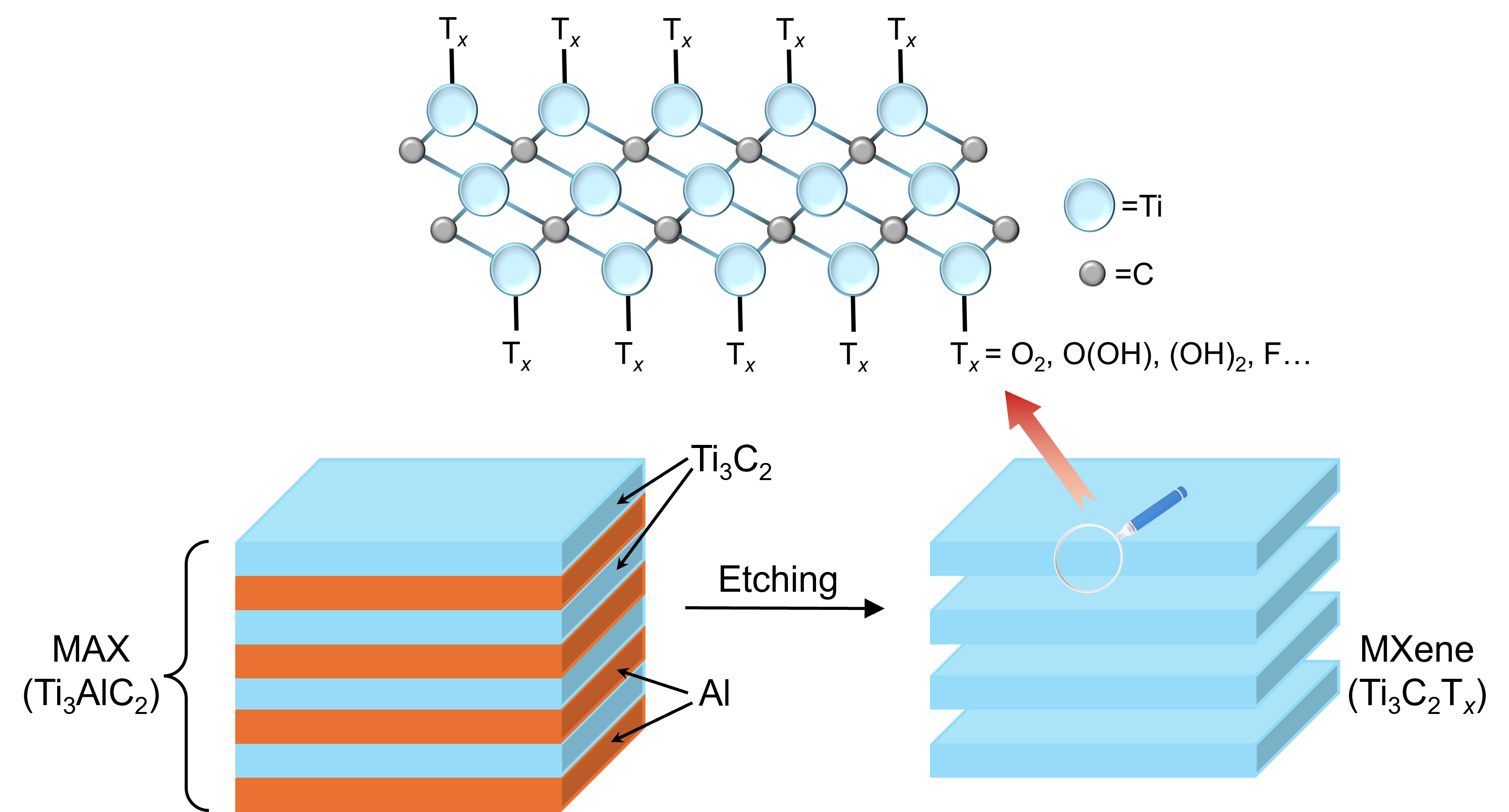
Figure 1. MXene preparation by MAX phase etching.
Since their discovery, interest in MXenes has rapidly increased, and this is reflected in a vast expansion in the number of potential applications being reported since 2011.3 These include:
MXenes possess unique combinations of properties, including amenable surface chemistry for tuneable functionality, high electrical conductivity, and mechanical strength. Furthermore, the high degree of modification of MXene structures, with numerous elements being able to be incorporated into the 2D structure as the M, X and Tx building blocks, means it is anticipated that in the near future, more complex MXenes will be synthesised with even more powerful properties.
Raman spectroscopy is an excellent technique for assessing MXene quality both during material synthesis and device fabrication. The method is highly sensitive to the structure and surface chemistry of MXenes, which are often highly variable when analysing bulk powders of the material. It excels in detecting different Tx species, alterations in the lattice structure, layer number, and the degradation of the material into transition metal oxides and amorphous carbon. It is ideal for monitoring synthesis procedures, assessing the structural integrity of the material, and probing interfacial reactions. In this Application Note, we demonstrate the high degree of sensitivity and specificity that Raman spectroscopy brings to the analysis of MXenes using the Edinburgh Instruments RM5 Confocal Raman Microscope.
The sample analysed was a Ti3C2Tx MXene powder dispersed in isopropyl alcohol (IPA). A 10 uL aliquot was dried onto a stainless steel microscope slide, which was then mounted onto the stage of the RM5, Figure 2. The crystals were then analysed using a 638 nm laser, a 600 gr/mm diffraction grating, and a 300 s acquisition time. Cosmic ray removal was performed on the spectra before they were baseline corrected and smoothed using a Savitsky-Golay filter with a 7-point window in Ramacle®.

Figure 2. Edinburgh Instruments RM5 Confocal Raman Microscope.
The critical region in the Raman spectrum of MXenes is between 100 cm-1 and 800 cm-1. This low-wavenumber window is rich in information regarding the presence of different Tx species, the number of layers, interlayer spacing, the temperature of the sample, and the degree of decomposition and oxidation within the crystal lattice. To add to this complexity, spectra recorded from the same MXene with different laser wavelengths or sample preparation procedures vary in the full-width half maxima and peak positions of various bands and the presence of pre-resonance Raman signatures.4,5 However, this spectral complexity means that Raman spectroscopy is useful for determining the properties of the material at various stages in the preparation of MXene-based technologies. Figure 3a shows the Raman spectrum of Ti3C2Tx MXene prepared by drop-casting a suspension in IPA and recorded using a 638 nm laser at room temperature.
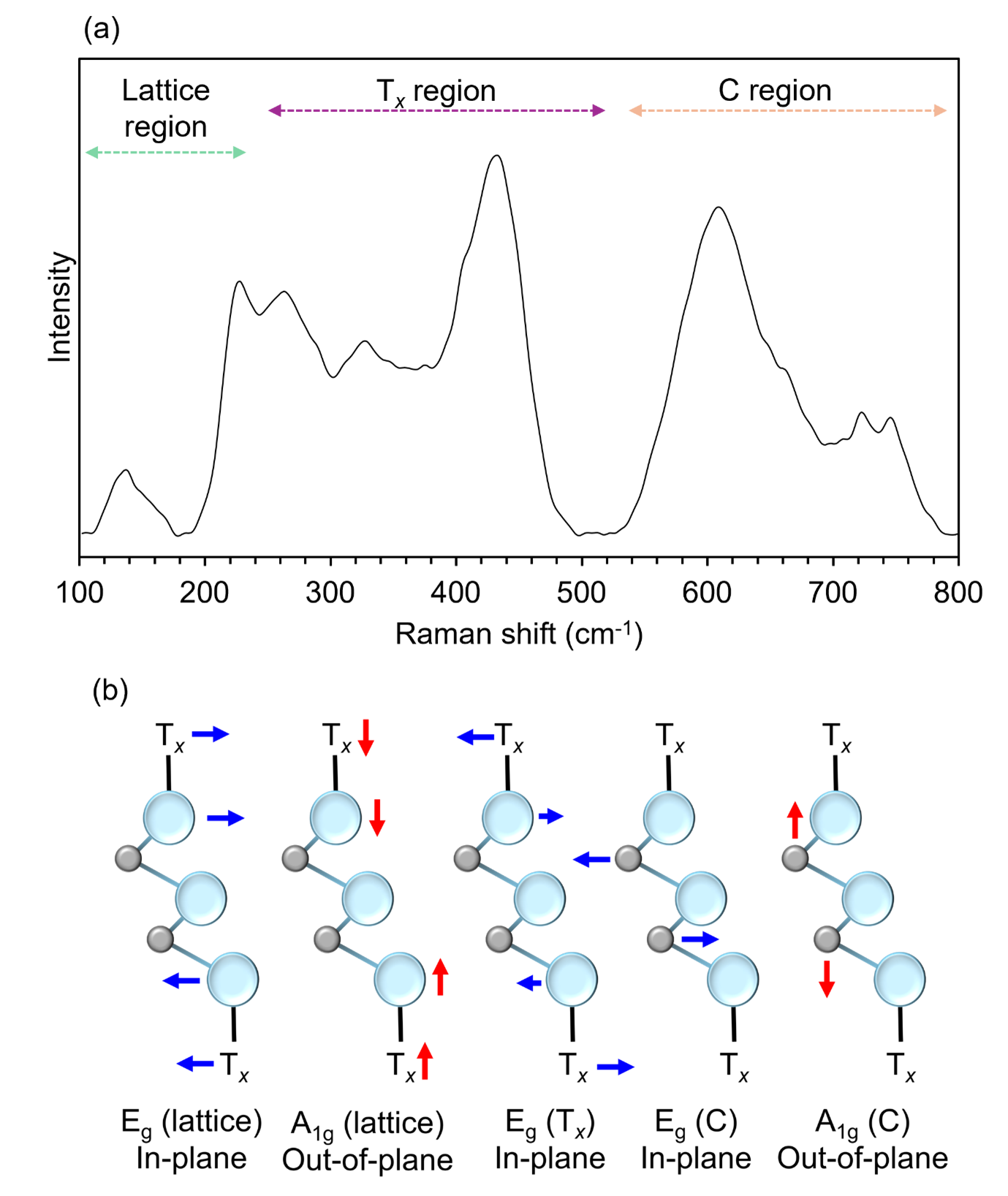
Figure 3. (a) Raman spectrum of Ti3C2Tx MXene and (b) illustration of Raman active modes.
The Raman spectrum of Ti3C2Tx MXene can be understood using the molecular symmetry of the lattice and the addition of different surface terminations on the lattice, Figure 3b. Ti3C2Tx belongs to the D3d point group, and the vibrations for such a lattice structure are described as 4Eg + 2A1g + 4Eu + 2A2u. Group theory predicts four Raman active modes: an Eg (in-plane) whole lattice vibration, an A1g (out-of-plane) whole lattice vibration, an Eg vibration of C atoms, and an A1g vibration of C atoms. There are also various additional Raman bands corresponding to surface terminations, of which there can be multiple. Each surface termination and intercalated species distort the Ti3C2 structure differently and influence the corresponding Raman lattice modes.
The modes corresponding to the whole lattice, Tx, and C vibrations all occur within different regions of the spectral region shown. The region between 100 cm-1 and 240 cm-1 can be assigned to whole lattice Eg and A1g vibrations. If decomposition of the MXene lattice occurs and titanium dioxide forms, then the main spectral signature of anatase at 150 cm-1 will become dominant, Figure 4. This can occur if the laser power applied to the sample is too high, so applying low powers and long acquisition times is crucial to acquire information about the material non-destructively.6 The Tx region between 250 cm-1 and 500 cm-1 corresponds to Eg vibrations of surface terminations, for example, fluorine atoms or hydroxyl functional groups attached to titanium atoms. This region provides information about the surface chemistry of MXenes and could be used for reaction-sensing applications. Finally, the bands within the 580 cm-1and 740 cm-1 region can be assigned to Eg and A1g carbon vibrations.
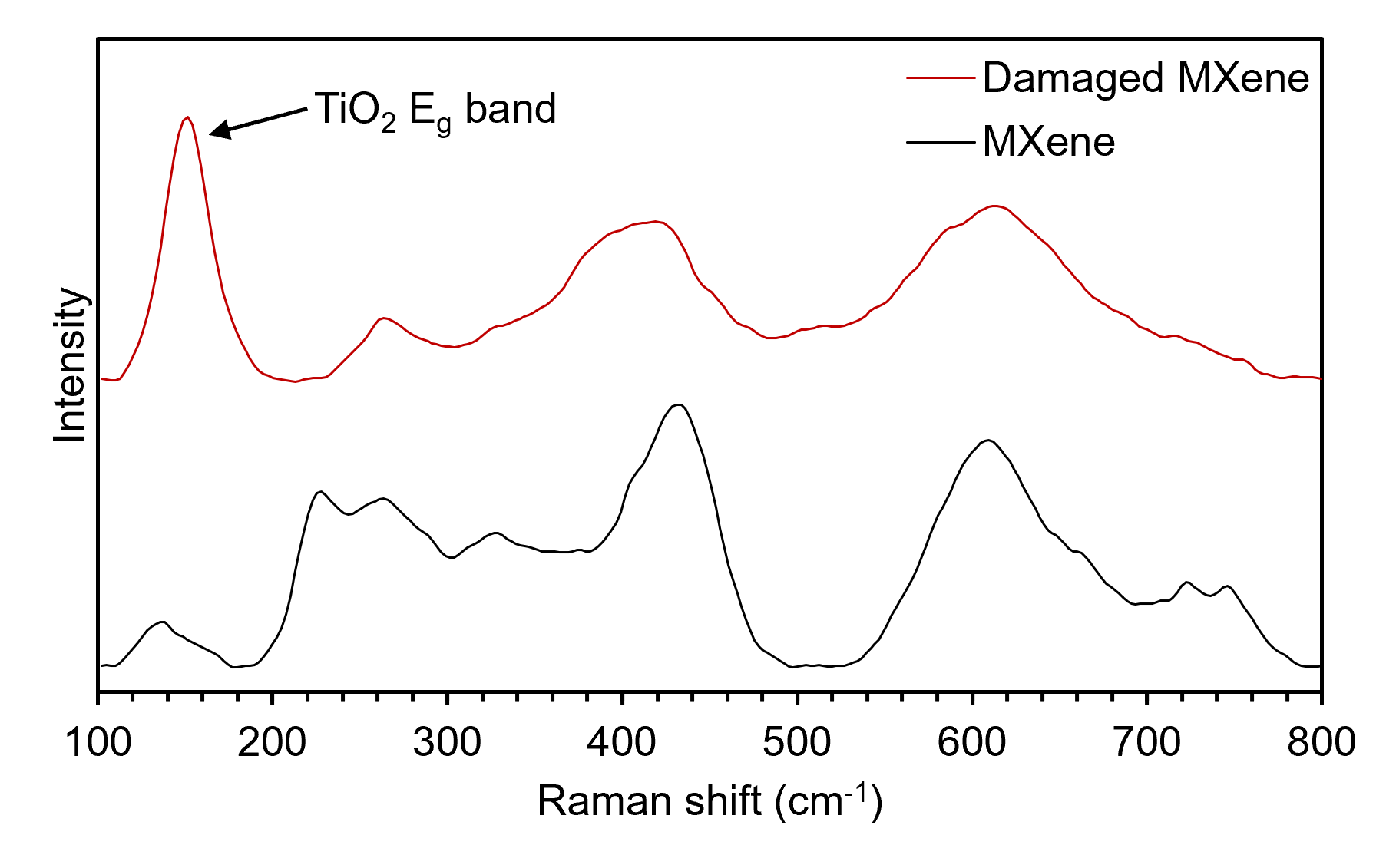
Figure 4. Raman spectrum showing the formation of anatase in a Ti3C2Tx MXene sample as a result of laser damage.
In this Application Note, the high degree of sensitivity and specificity that Raman spectroscopy brings to the analysis of MXenes was demonstrated. Raman spectroscopy can detect the presence of different Tx species, alterations in the lattice structure, layer number, and the degradation of the material into transition metal oxides and amorphous carbon. Therefore, it is an excellent technique for assessing MXene quality during material synthesis and device fabrication.