Understanding and optimising novel materials for the next generation of fuel sources for our energy needs that are efficient, affordable, and carbon neutral is of paramount importance for generations to come. Photocatalytic materials that can harvest sunlight and generate hydrogen gas (H2) from water is highly sought after given the enormous energy released and environmentally friendly by-product of water when burning this gas for energy. However, this process requires a material that can undergo a series of electron transfers to generate the electrical potential needed to generate H2 from water molecules and be stable enough to continue production cycles. Metal-organic frameworks (MOFs) are one such material that exhibit these properties, as their porous crystalline nature of metal ions and organic linkers offers the ability to tune the electrical potential, generate large absorption extinction coefficients, and rigid stability. In order to design and optimise MOFs as photocatalysts, the measurement of energy and electron transfer rates and detecting the excited state species present are decisively essential; the Edinburgh Instruments LP980 Transient Absorption Spectrometer and the Mini-Tau Fluorescence Lifetime Spectrometer are expertly designed to facilitate this type of research to ensure the goal of a carbon neutral future (Figure 1).

Figure 1. The Edinburgh Instruments LP980 Transient Absorption Spectrometer (top) and the Mini-Tau Fluorescence Lifetime Spectrometer (bottom).
A group of scientists from Madrid, Spain’s IMDEA Energy Institute and the Instituto de Ciencia de Materiales-CSIC synthesised and evaluated an innovative bismuth-based MOF they named IEF-5 (IMDEA Energy Framework-5) as a photoelectrode in a H2 generating photocatalytic reaction. This work, funded for Horizon 2020 by a European Research Grant though an ERC-CoG (HYMAP, No. 648319), utilised multiple spectroscopies, including transient absorption and fluorescence lifetime measurements from an Edinburgh Instruments LP980 and Mini-Tau Spectrometers, respectively, to map the photocatalytic pathways during H2 production, while also determining the amount of H2 production, material redox potentials, and ultrafast charge separation by core hole clock synchrotron time-resolved methods.
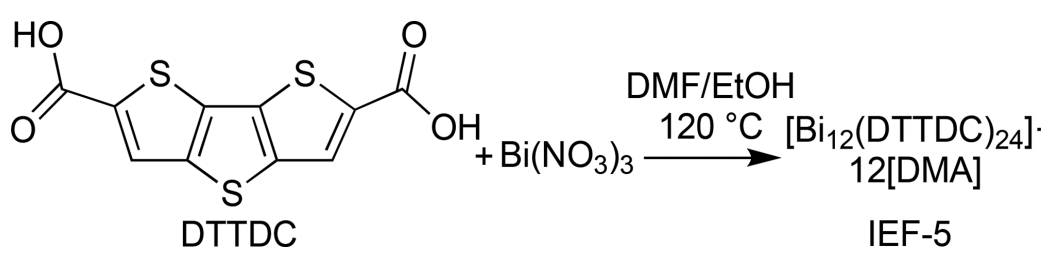
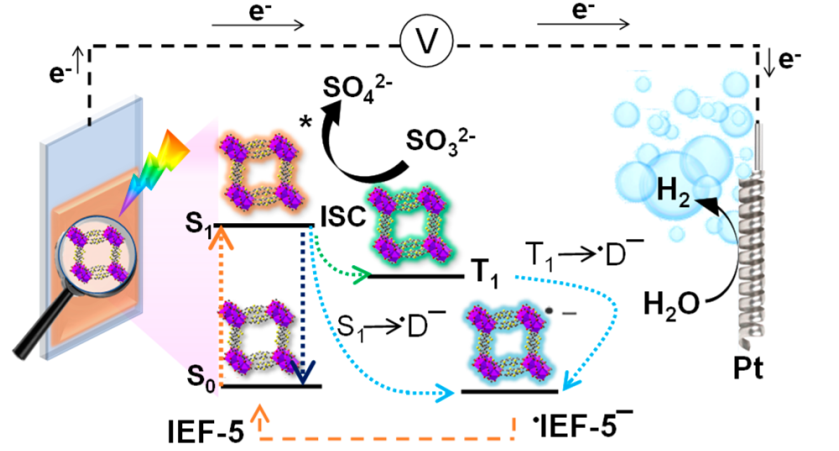
Figure 2. (Top) The synthesis of a novel bismuth-based MOF (IEF-5) and (Bottom) its photocatalytic H2 production cycle.
Figure 2 illustrates the photocatalytic H2 production cycle of IEF-5 solvated in an electrolytic, electron donating solution. In the presence of light activation, both excited singlet 1IEF-5* and triplet 3IEF-5* are formed, with the latter happening through intersystem crossing (ISC). Both excited singlet and triplet states interact with the electrolytic solution’s hole scavenger, SO32-, injecting charge into the MOF forming a radical anion ·IEF-5–, that then shuttles the harnessed electron to the Pt-cathode for continued cycling of the H2 production mechanism to evolve H2 gas. The hole acceptor subsequently retains the leftover oxygen atom to balance the reaction.
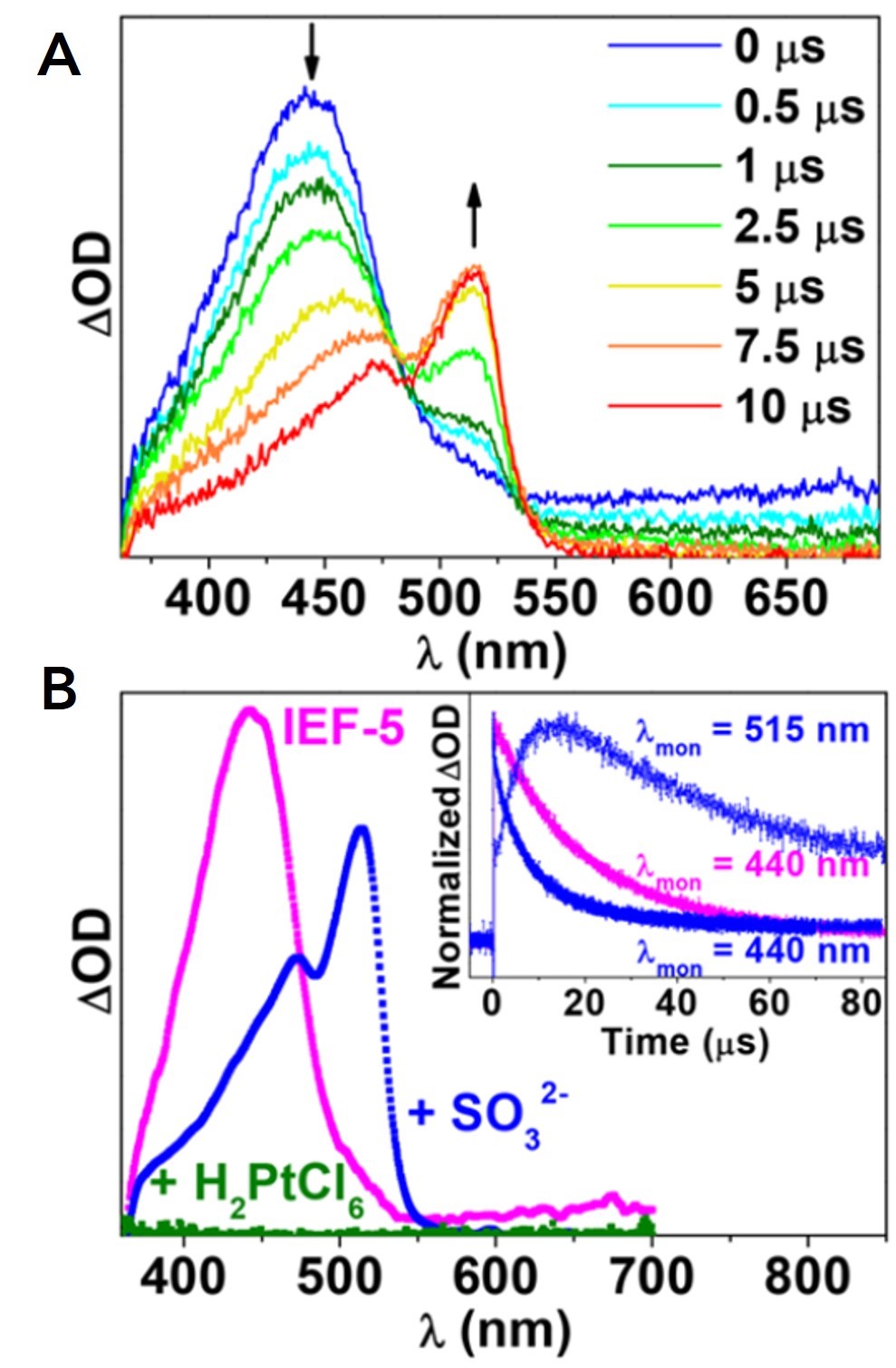
Figure 3. (A) Transient absorption spectral evolution of IEF-5 in the presence of 0.5 M Na2SO3, and (B) transient absorption of IEF-5 (magenta), and IEF-5 in the presence of an electron acceptor H2PtCl (green) and electron donor Na2SO3 (blue) with colour corresponding lifetime traces (B inset). The excitation wavelength was 355 nm for these data sets.
The IEF-5 photocatalytic mechanistic details were gleaned from the clever use of transient absorption spectra and lifetimes in the presence of an electron donor (Na2SO3) and electron acceptor (H2PtCl). Figure 3A shows the time-gated spectral transients of IEF-5 in the presence of 0.5 M Na2SO3 measured utilising the LP980’s ICCD camera. Comparing these spectra to the transient absorption of just IEF-5 and IEF-5 with the electron donor Na2SO3 (Figure 3B), there is direct observation of the formation of an excited IEF-5* in the early times transitioning to the formation of ·IEF-5– in the presence of SO32- in later times. Comparing the lifetimes with and without the Na2SO3 electron donor (Figure 3B inset), as well as further studies into concentration dependent excited state lifetimes of IEF-5 and Na2SO3, showed a clear Stern-Volmer correlation for reaction rates.
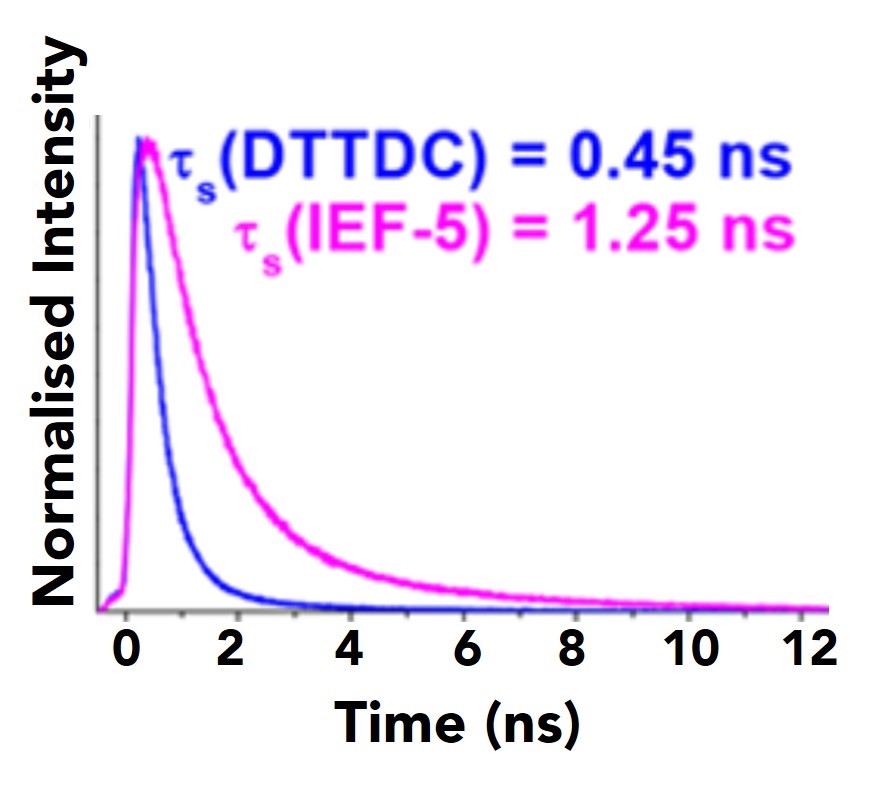
To gain further insights into IEF-5 excited states, fluorescence lifetimes of the DTTDC base molecule and its IEF-5 MOF counterpart revealed almost a three-time increase in lifetime for the IEF-5. This is likely attributed to ligand-to-ligand charge transfer when compared to TD-DFT calculations, though could also be liable to early ligand-to-metal charge transfer (LMCT).
Leveraging novel photocatalytic materials for H2 fuel production presents significant advantages over fossil fuels in terms of stored energy and environmental impact that will change our future for the better. Understanding the mechanisms associated with energy production will undoubtedly lead to the next generation of devices; the Edinburgh Instruments portfolio of spectrometers for transient absorption and photoluminescence spectrometers are perfectly suited to conduct and improve these research goals.
https://pubs.acs.org/doi/10.1021/jacs.9b10261
Figures reprinted with permission from J. Am. Chem. Soc. 2020, 142, 318-326.