Studying phase transitions in food science is indispensable for optimising product quality, stability, and sensory attributes. For example, the phase transition affects shelf life and product storage whilst also impacting the bioavailability of nutrients in food. Food texture undergoes significant changes due to phase transitions with the melting characteristics of fats and oils playing a pivotal role in determining the smoothness of products such as butter.
Butter is a water-in-oil emulsion primarily composed of water, milk fat, and milk solids. The concentration of the main ingredients vary depending on the desired consistency of the butter, for example, between spreadable and non-spreadable butter. Spreadable butter contains a higher percentage of unsaturated fats and added oils compared to traditional non-spreadable butter. This lowers the melting point of the butter, making it softer and more spreadable. Non-spreadable butter has a firmer consistency and is solid at room temperature with no oil additives present. Spreadable butter has gained popularity for its ease of use, whilst traditional butter is more commonly used in baking, where additional oils could detrimentally affect the end product.
Here, the IR5 FTIR Spectrometer is used to demonstrate the versatility and practicality of FTIR spectroscopy in the field of food science and technology. Specifically, when combined with a heated Attenuated Total Reflectance (ATR) accessory it highlights the instrument’s capability to analyse complex lipid systems like butter, discerning phase transitions from solid to separated oil and water phases. Studying molecular composition allows a greater understanding of factors which influence butter texture, stability, and functionality; which facilitates the optimisation of butter products for their intended use.

Figure 1: Edinburgh Instruments IR5 FTIR Spectrometer
Two butter products (Table 1) were studied using an IR5 fitted with a heated diamond ATR accessory. 0.5 g of each sample was placed onto the heated ATR crystal and subsequently heated from 25°C to 65°C, and spectra were collected at every 5°C step. The FTIR spectra were collected with 10 scans per spectrum at a resolution of 4 cm-1.
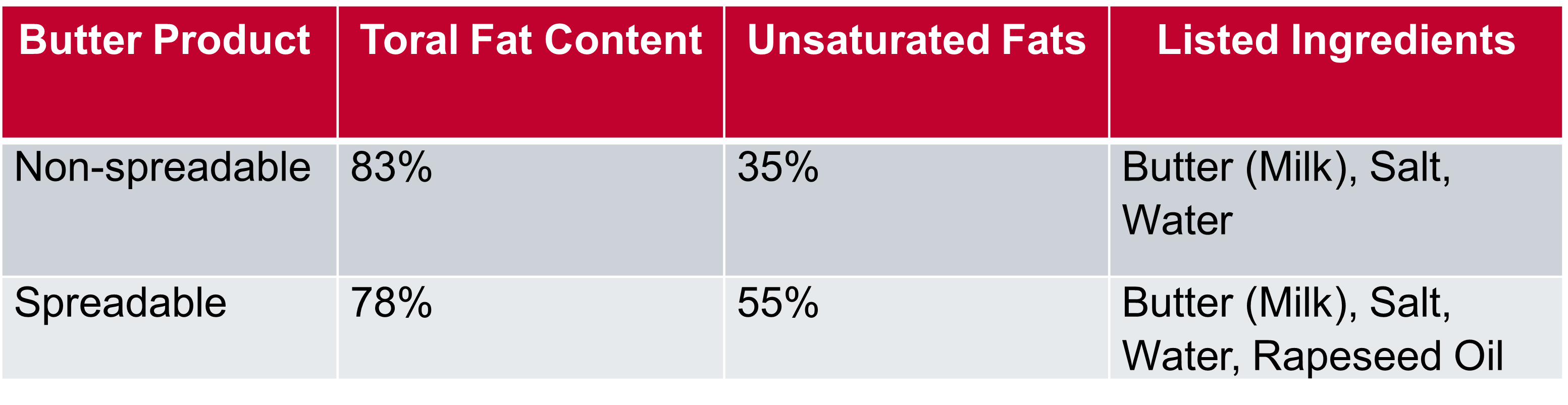
Table 1: Butter Samples
An FTIR spectrum from traditional non-spreadable butter at room temperature is shown in Figure 2. The spectrum is dominated by peaks associated with oil components in the butter emulsion, for example, 1750 cm-1 from C=O present in esters, and the peaks from 3000-2800 cm-1 associated with C-H stretches from fatty acids.
Figure 2: FTIR spectrum of non-spreadable butter with band assignments 1
Table 2: Band Assignments of non-spreadable butter 1
When heating the non-spreadable traditional butter from 35°C to 65°C it separates into two phases. A water phase remains on the bottom and an oil phase rises to the top of the sample, Figure 3. ATR-FTIR has a small depth of penetration, as the butter melts and the phases split the ATR-FTIR spectra will be dominated by water phase bands.
Figure 3: Butter sample on ATR crystal at room temperature (a) and at 65°C (b).
Figure 4 shows the spectral differences when heating the sample, there is a clear distinction between the emulsion and water phases. The transition between phases can be seen from a gradual decrease of oil phase bands, partnered with rapid increasing of water phase bands. Within the water phase there are also some proteins from the milk solids, which account for the subtle spectral peaks at 1530 cm-1 and 1035 cm-1, noted with an asterisk.
Figure 4: Non-spreadable butter temperature study.
The spreadable butter was also studied with the temperature ATR accessory, where even at room temperature, there were spectral indications of water from the high wavenumber O-H stretching band (~3400 cm-1). The two phases were fully separated at 35°C, showing the much lower phase transition temperature in spreadable versus non-spreadable butter. This is expected with the addition of rapeseed oil to the sample and its higher concentration of unsaturated fats. This is because unsaturated fats have more carbon-carbon double bonds, and when there are a higher number of these double bonds present from unsaturated fatty acids, the melting point will be lower.
Figure 5: Spreadable butter temperature study.
The composition of butter affects its end use for the customer, i.e., whether it be used on toast and need to be easily spread, or for baking a cake. Adding oils to butter makes it spreadable and decreases the phase transition temperature. Temperature controlled ATR-FTIR spectroscopy is an ideal technique to study phase transitions in food science thanks to the wealth of molecular information obtained.