Fluorescence spectroscopy reveals the light-emitting secrets of molecules and materials. By exciting a sample with specific wavelengths, we measure the emitted light’s wavelength and timescale. Discover how our photon counting steady-state and time-resolved fluorescence spectrometers can elevate your research.
What is Fluorescence Spectroscopy?
Fluorescence (photoluminescence) spectroscopy studies the light emitted by molecules and materials. There are two main types of fluorescence spectroscopy: steady-state and time-resolved.
Steady State Fluorescence Spectroscopy
In steady-state fluorescence, the sample is excited by a constant excitation light source, and the intensity and wavelength of the emission are measured.
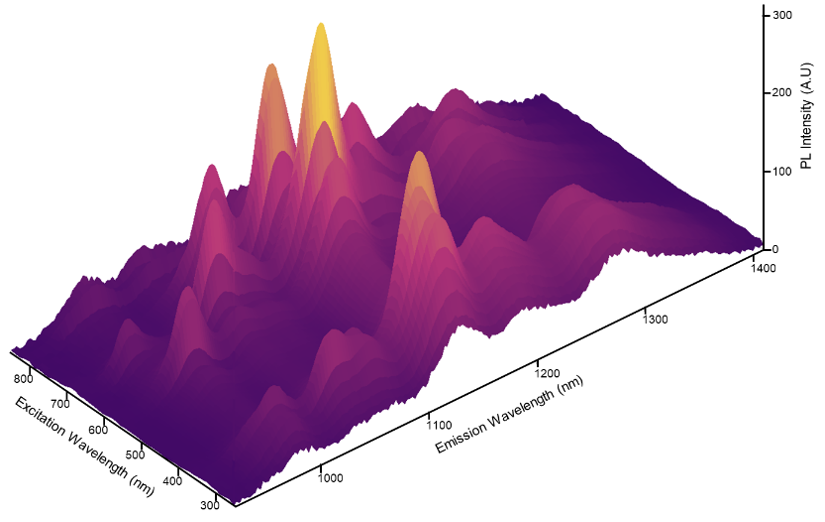
Most often, this is a fluorescence emission spectrum, which shows how the intensity of emitted light changes with wavelength. The peak wavelength and shape of the spectrum reveal information about the sample's electronic energy levels.
Often, the shape and intensity of the spectrum will change with variables such as concentration, solvent polarity, temperature, and pH, revealing additional information. Other commonly used types of steady-state fluorescence spectroscopy include excitation spectra, excitation-emission maps, and quantum yield.
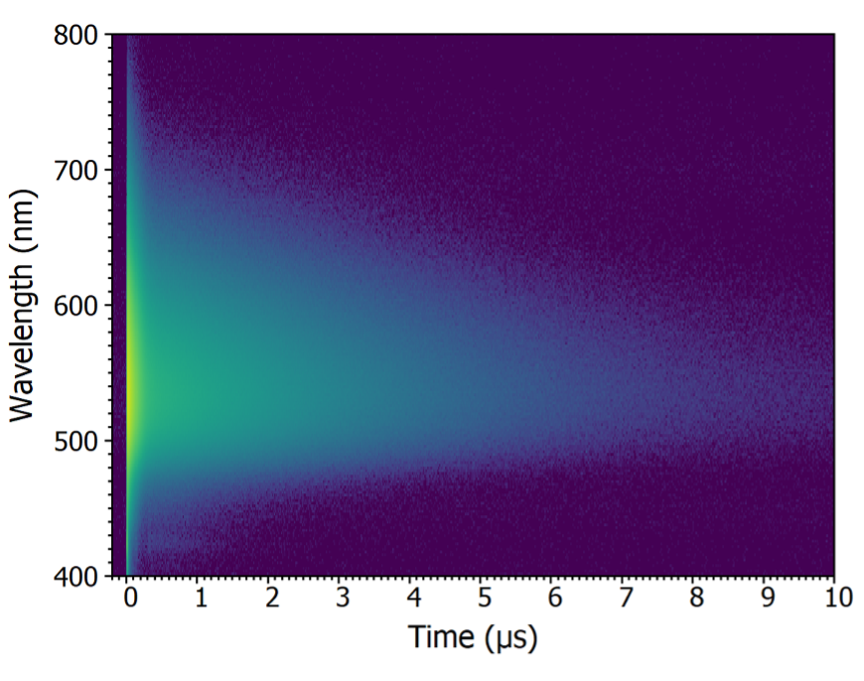
Time-Resolved Fluorescence Spectroscopy
In time-resolved fluorescence, the sample is excited by a pulsed light source and the timescale of the light emission is studied.
For time-resolved measurements, it is important to distinguish between fluorescence emission, which occurs on a picosecond to nanoseconds timescale and slower phosphorescence, which lasts microseconds to seconds. The distinct timescales require different measurement techniques. Time-correlated Single Photon Counting (TCSPC) is ideal for fluorescence, and Multichannel Scaling Photon Counting (MCS) for phosphorescence.
Both techniques measure the lifetime of the emission, which is the average time that a molecule or material stays in the excited state before emitting light.