In the broadest sense a spectrometer is any instrument that is used to measure the variation of a physical characteristic over a given range; i.e. a spectrum. This could be a mass-to-charge ratio spectrum in the case of a mass spectrometer, the variation of nuclear resonant frequencies in an NMR spectrometer or the change in the absorption and emission of light with wavelength in an optical spectrometer.

Figure 1: The three most common types of spectrometers found in research labs around the world. Left: Optical spectrometer (Edinburgh Instruments FS5 spectrofluorometer). Centre: NMR spectrometer (Agilent 800 MHz NMR spectrometer). Right: Mass spectrometer (Scion Instruments GC-MS spectrometer).
The most ubiquitous type of spectrometer used for research are optical spectrometers; and when someone simply says ‘spectrometer’, without an additional qualifier, they are usually referring to an optical spectrometer and this diverse family of spectroscopic instruments is the focus of this article.
The goal of any optical spectrometer is to measure the interaction (absorption, reflection, scattering) of electromagnetic radiation with a sample or the emission (fluorescence, phosphorescence, electroluminescence) of electromagnetic radiation from a sample. Optical spectrometers are concerned with electromagnetic radiation that falls within the optical region of the electromagnetic spectrum which is light spanning the ultraviolet, visible and infrared wavelength regions of the spectrum.
In order to gain maximum information, the interaction or emission of light should be measured as a function of wavelength and the common feature of all optical spectrometers is therefore a mechanism for wavelength selection. In low cost spectrometers or in situations where accurate wavelength selection is not important, optical filters are used to isolate the wavelength region of interest. However, for accurate wavelength selection and the generation of spectra, a dispersive element that separates light into its constituent wavelengths is required. In all modern spectrometers, this dispersive element is a diffraction grating where constructive and destructive interference is used to spatially separate polychromatic light that is incident on the grating (Figure 2).

Figure 2: Dispersion of light into its constituent wavelengths by a diffraction grating.
Diffraction gratings are a key component of a monochromator, which is a device used to select a particular wavelength of light from a polychromatic light source. In a monochromator the diffraction grating is rotated to change the wavelength that aligns with and passes through the exit slit. Excitation monochromators are found in all spectrophotometers (see following section) for selecting the desired excitation wavelength to reach the sample from a white light source (Figure 3 Excitation Monochromator) and spectra are measured by scanning the monochromator and measuring the change in signal as a function of excitation wavelength.
For detecting the light emitted by a sample there are two approaches. The first is an emission monochromator which works using the same principle as above except the light source is the emission from a sample and the monochromator selects which wavelength of light reaches the detector (Figure 3 Emission Monochromator). The second approach is to detect the spectrum of the dispersed light ‘all at once’ using an array detector (such as a CCD camera) which is called a spectrograph (Figure 3 Spectrograph). At least one emission monochromator or spectrograph is found in all spectrofluorometers and Raman spectrometers (see following sections).

Figure 3: The basic operating principle behind monochromators and spectrographs. It should be noted that these images are highly simplified for illustration. For example the monochromators used in the Edinburgh Instruments FS5 and FLS1000 are a more complicated Czerny-Turner design which have two slits and two ellipsoidal mirrors for superior performance but the principle remains the same.
Now that the key component of a spectrometer has been identified, the different types of spectrometer, their role, and basic design can be discussed. Three of the most common optical spectrometers: spectrophotometers, spectrofluorometers and Raman spectrometers are introduced.
The term spectrophotometer can refer to quite a variety of instruments that measure light, with the exact definition depending on the area of science or industry. In all cases the term ‘photo’ is used to indicate that the spectrometer is for the quantitative measurement of light intensity with wavelength. Within academic research (particularly chemistry and biology laboratories) the term spectrophotometer is used specifically to refer to a spectrometer which measures the absorption of light by a sample and that definition will be used here.

Figure 4: Simplified diagram of a single beam spectrophotometer.
A stylised layout of a basic single beam spectrophotometer is shown in Figure 4. It comprises a white light source which is usually either a combination of a deuterium arc lamp to cover the UV range and a tungsten halogen lamp to cover the visible; or a single Xenon arc lamp to cover the entire range. This is followed by an excitation monochromator which selects the wavelength of light that reaches the sample. This light is then either transmitted through the sample (as shown in Figure 4) for transparent samples such as solutions or reflected off the surface for opaque samples. The intensity of the transmitted or reflected light is then monitored using a detector which is typically a photomultiplier tube or silicon photodiode.
In addition to stand alone spectrophotometers, the functionality of a spectrophotometer can also be incorporated as a secondary feature into other spectrometers. An example of this is the FS5 Spectrofluorometer which comes with a transmission detector as standard and has all the functionality of a single beam spectrophotometer in addition to being a spectrofluorometer.
The most common measurement undertaken in a spectrophotometer is measuring the absorption spectrum of a sample. The excitation monochromator is scanned and the change in light intensity transmitted through the sample recorded on the detector. This is then repeated with a reference sample and the absorption spectrum calculated as shown in Figure 5 for a solution of fluorescein in phosphate buffered saline.

Figure 5: Absorption spectrum of fluorescein measured using the FS5 Spectrofluorometer.
A more advanced version of a spectrophotometer is a transient absorption spectrometer, which can measure the evolution of the absorption spectrum with time and is essential for looking at temporary species generated through chemical reactions or short lived photoexcited states. The operation and design of transient absorption spectrometers are beyond the scope of this article but you can learn more in our introduction to transient absorption using the Edinburgh Instruments LP980 Transient Absorption Spectrometer.

Figure 6: Edinburgh Instruments LP980 Transient Absorption Spectrometer.
A spectrofluorometer is used to measure the fluorescence emission (or more generally, the photoluminescence) from a sample. The terms spectrofluorometer and fluorescence / photoluminescence spectrometer are interchangeable and different manufacturers call them by different names. A general convention is that spectrofluorometer refers to a compact benchtop instrument that is similar in size to a spectrophotometer, such as the FS5 Spectrofluorometer, while the term fluorescence / photoluminescence spectrometer is used for larger spectrometers with superior performance and more diverse functionality such as the FLS1000 Photoluminescence Spectrometer.

Figure 7: Example of a compact spectrofluorometer (FS5) and a more advanced photoluminescence spectrometer (FLS1000).
The layout of a typical steady state spectrofluorometer is shown in Figure 8. The excitation side of a spectrofluorometer is equivalent to the spectrophotometer: a white light source and an excitation monochromator. Xenon arc lamps are used as the light source as their high brightness is essential to measure the weak fluorescence emission. The sample is illuminated by the chosen excitation wavelength which causes it to fluoresce. The fluorescence emission is collected by the emission monochromator which is orientated at 90 degrees to the excitation monochromator and the selected wavelength reaches the detector; typically a photomultiplier tube. For more detail on the design of spectrofluorometers read our article on fluorescence instrumentation.

Figure 8: Simplified diagram of a spectrofluorometer.
The two most common spectral measurements undertaken in a spectrofluorometer are excitation and emission spectra. To measure an excitation spectrum the emission monochromator is set at a wavelength of strong fluorescence emission, the excitation monochromator is scanned across the wavelength region of interest and the change in fluorescence intensity monitored on the detector (Figure 9 blue curve) which reveals the absorption features of sample. Emission spectra are the inverse of this; the wavelength of the excitation monochromator is set where there is strong absorption by the sample, the emission monochromator is swept across the wavelength region of interest and the change in fluorescence detected (Figure 9 red curve) revealing the fluorescence features of the sample.

Figure 9: Fluorescence excitation (blue) and emission (red) spectra of anthracene measured using the FS5 Spectrofluorometer.
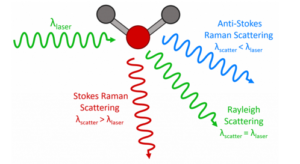
Raman spectrometers are used to measure the Raman scattering of light from a sample. The design of a typical Raman spectrometer is shown in Figure 10 and is similar to a spectrofluorometer but with a few key differences. The white light source and excitation monochromator found in spectrofluorometers are replaced with a laser. The reason for this is twofold. The first is that ‘Raman’ is a scattering effect and the light is therefore not absorbed by the sample. This means that a broadband tuneable light source for matching to the absorption features is not required. The second reason is that the Raman effect is much weaker than fluorescence (ratio of Rayleigh scattered light to Raman scattered light is ~106) and sources with a high photon flux are therefore essential to maximise the signal.

Figure 10: Simplified diagram of a Raman spectrometer.
The laser light is scattered off the sample and passed through a filter to remove the much more intense Rayleigh scattered component. The remaining Raman scattered light is then passed into a spectrograph and captured using a CCD detector to give a Raman spectrum like that shown in Figure 11 of paracetamol.

Figure 11: Raman spectrum of paracetamol.
If you have enjoyed reading this blog post about our spectroscopic instruments, and want to be the first to see all the latest news, applications and product information, then sign-up to our infrequent newsletter via the red sign-up button below and join us on social media.